Breaking News
Researchers re-engineered the structure of the commonly ussed chemotherapy drug 5-fluorouracil (5FU) into that of a “spherical nucleic acid” — a nanostructure that weaves the drug directly into DNA strands coating tiny spheres, making its cancer cell-killing ability 20,000 more effective, while also reducing its toxicity.
A new study by researchers at Swansea University has revealed a new way to potentially treat certain autoimmune diseases by targeting a protein that helps regulate energy production in immune cells.
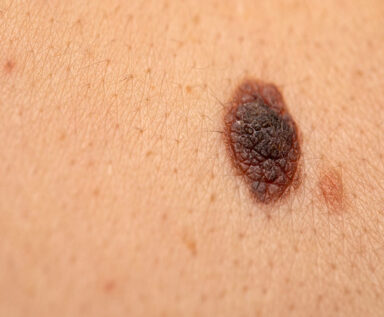
Early data from a Phase II clinical trial show a combination of immunotherapy medications can activate a robust immune response and help overcome treatment resistance in patients with refractory melanoma.
BioTrending
- By Keith Berman, MPH, MBA
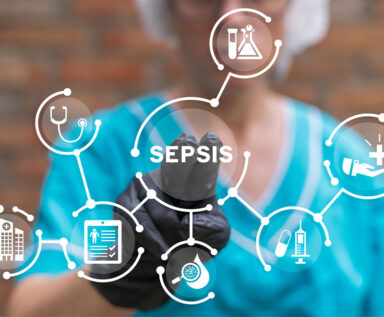
Therapeutic plasma exchange may improve outcomes for patients diagnosed with sepsis or septic shock, but funding is needed to conduct needed clinical trials.
- By Trudie Mitschang
As chronic disease and aging populations drive healthcare costs higher, value-based care offers a sustainable path forward — rewarding providers for better outcomes, improved patient experiences and greater cost-efficiency.
- By Diane L.M. Cook
With a pending shortage of cardiologists, there is a growing need for remote cardiac monitoring, and now, three FDA-cleared products are transforming how this care is provided.
- By Lee Warren
The use of AI is growing at a brisk rate as healthcare professionals realize its potential as an indispensable partner in treating patients.
Awards
BSTQ is an Industry Award-Winning Journal
Since its debut in 2009, BioSupply Trends Quarterly’s articles have been recognized by some of the industry’s most prominent organizations, earning platinum and gold awards.