Flublok: A Potent New Weapon Against the Shape-Shifting Influenza Virus
- By Keith Berman, MPH, MBA
RAPID GENETIC MUTATION is the evolutionary strategy that, year after year, enables influenza viruses to evade antibody-mediated defenses and newly infect people who have had the flu or received flu immunizations many times in the past. A “universal” flu vaccine that can provide broad multi-seasonal protection against new emergent strains by targeting conserved antigenic sites on the virus still exists only in our imaginations. Our best line of defense remains immunization with new flu vaccines prepared annually against emergent influenza strains identified as the likeliest to produce the next seasonal flu epidemic.
But its propensity to continually mutate makes the influenza virus a moving target for vaccine manufacture. Mutations in the gene encoding hemagglutinin (HA) — the surface glycoprotein antigen against which we mount an antibody response — reduce the match between surface HA antigen in virus strains selected each February by the World Health Organization (WHO) and HA antigen that appears on circulating epidemic strains that finally arrive in the fall and winter. This “antigenic drift” in turn reduces vaccine effectiveness.
Atop gene mutations caused by “antigenic drift” are additional mutations introduced by genetic manipulations of selected vaccine virus strains to produce “high-growth reassortments” that yield more HA antigen in embryonated chicken eggs. In the 2016-2017 flu season, egg-based flu vaccines accounted for about 95 percent of the roughly 160 million doses distributed in the U.S. last year.
In flu seasons when epidemic influenza strains are more closely matched to the WHO-selected vaccine strains, immunization can reduce the risk of contracting laboratory-confirmed influenza by as much as 50 percent to 60 percent. But other years when more mutations occur due to combinations of antigenic drift and egg-adaptive reassortment, flu vaccine effectiveness can drop precipitously (Figure 1). The relationship is brutally straightforward: the poorer the genetic match between the selected vaccine strains and the strains that become epidemic that fall and winter, the less protection it confers against influenza attack and the higher the incidence of flu-related illness, hospitalizations and death.
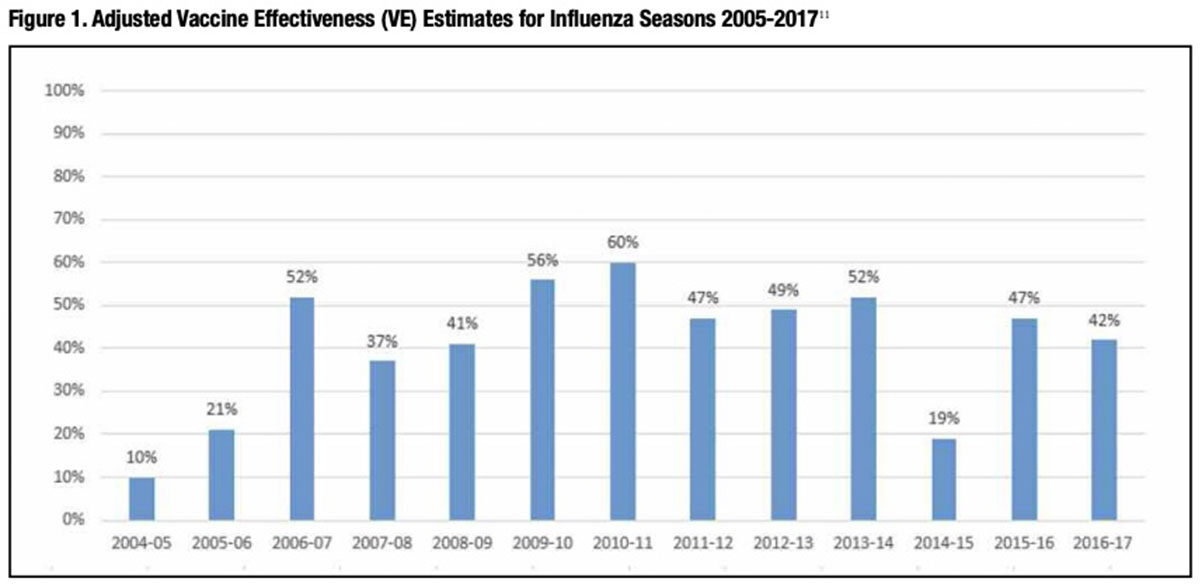
Entirely apart from vaccine potency, impaired or declining immune function of the vaccine recipient can also account for diminished vaccine protection. To boost flagging vaccine responsiveness in persons aged 65 years and older — the age group that also accounts for most flu-related deaths — Sanofi Pasteur introduced Fluzone High-Dosein 2009, an inactivated influenza vaccine (IIV) featuring four times the standard 15 micrograms (μg) of HA antigen for each A and B strain. In 2015, the U.S. Food and Drug Administration (FDA) approved the first adjuvanted IIV, Seqirus’ Fluad, also indicated for persons aged 65 years and older. Both represent important innovations proven to reduce the incidence of influenza-like illness in older adults. But just like standard-dose non-adjuvanted IIVs prepared with the same candidate vaccine virus strains, their protective benefit is much diminished in flu seasons when there is a poor antigenic match between vaccine strains and circulating flu strains.
Could we one day see an influenza vaccine that is both highly and more consistently immunogenic against seasonal influenza virus, and thus able to provide better year-to-year protection against the ravages of the disease? Backed by an expanding body of evidence, there is good reason to believe that such a vaccine already exists. It is Protein Sciences’ Flublok, a recombinant quadrivalent product recently approved for adults aged 18 years and older.
More Antigen Boosts Flublok Immunogenicity
In persons 65 years of age and older, it is well documented that standard dose IIV induces a less-robust neutralizing antibody response than in younger adults.1 A large randomized trial of Fluzone High-Dose proved that simply increasing the administered dose of each HA antigen by four-fold, from 15 μg to 60 μg, both boosts hemagglutinin inhibition titers and reduces the influenza attack rates in seniors.2 Flublok is formulated with 45 μg of recombinant HA antigen per strain, three times the quantity in standard-dose IIVs.* Pre-licensure hemagglutinin inhibition studies in subjects 65 years of age and older demonstrated significantly higher seroconversion rates and higher postvaccination geometric mean titers (GMT) for both the A/H1N1 and A/H3N2 subtypes following immunization with Flublok compared to immunization with a standard egg-based IIV (Fluzone).3 Flublok was approved for use in 2013 based on its excellent immunogenicity and safety profile.
Earlier this year, results of a double-blind, multicenter trial finally addressed whether Flublok’s superior immunogenicity profile compared to standard-dose IIV translates into better flu protection.4 Just over 9,000 U.S. adults aged 50 years and older were randomized to receive the new quadrivalent form of Flublok or standard-dose egg-based quadrivalent IIV (IIV4) (Fluarix Quadrivalent, GlaxoSmithKline). Flublok immunization was associated with a 30 percent reduction in the cumulative incidence of laboratory-confirmed influenza-like illness (ILI) compared to IIV4 throughout the influenza season (95% confidence interval, 10 to 47; P=0.006) (Figure 2).
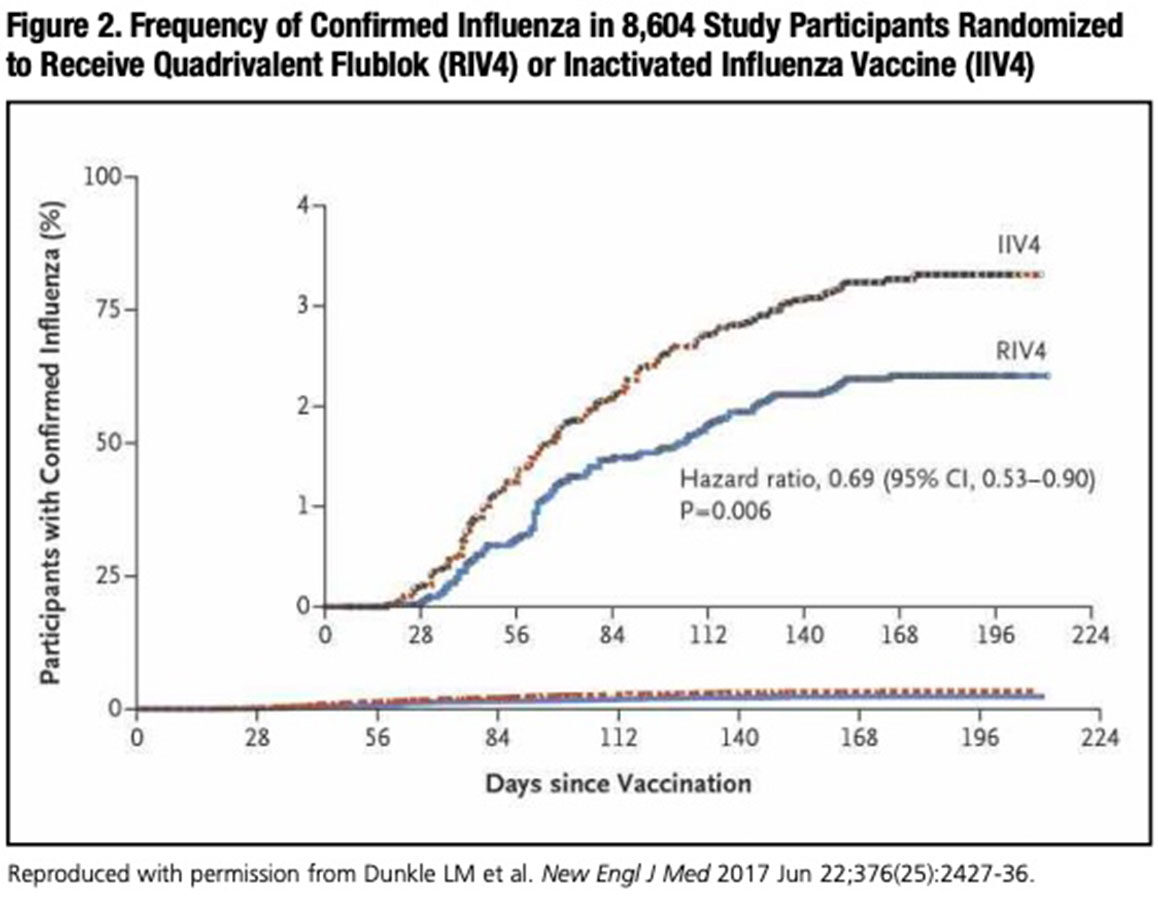
Surprisingly, in this study’s younger 50- to 64-year-old age cohort, the incidence of ILI in participants receiving quadrivalent Flublok was 42 percent lower than in those vaccinated with IIV4 — comparable to the 30 percent overall reduction for all participants age 50 years and older. While high-dose IIV has been shown to be marginally more immunogenic than standard-dose IIV in the 50- to 64-year-olds,5 no previous study has documented improved protection of high-dose vaccine against contracting ILI in the middle-aged adult population. Yet to be answered is what accounts for Flublok’s superior protection against ILI, including the 50- to 64-year-old age cohort in whom standard-dose IIV can still induce a reasonably robust antibody response.
Flublok and the Hemagglutinin (HA) Stalk Domain
The large majority of influenza-related deaths — up to 90 percent — occur in persons older than 65 years of age, the result of immunosenescence, as well as a higher prevalence of existing comorbidities. Yet, paradoxically, older adults have much lower flu attack rates than younger adults and children. A recent study in the Netherlands, for example, found that the A/H1N1 attack rate was by far the highest in children (35 percent), lower in adults age 20 to 39 years (6.6 percent) and lower yet in adults age 40 and older (2.8 percent).6
The puzzle of lower influenza attack rates in seniors might be explained in part by a very recent finding that titers of broadly neutralizing IgG antibodies against the conserved stalk region of HA are very low in children, but increase with age.7 Likely because the stalk domain of the HA glycoprotein is embedded in the viral membrane, egg-based seasonal flu vaccines usually do not induce these types of antistalk antibodies at significant levels. Further, while possibly less potent than antibodies against the exposed globular HA head, stalk-reactive antibodies have been shown to confer robust antivirus protection in vivo. Thus, some researchers speculate that the lower influenza attack rate with advancing age may at least in part be the result of repeated exposures to influenza viruses over many years, which prime broad protective antibody-based immunity against the conserved HA stalk domain of new seasonal virus strains.
As conventional split-virion IIV vaccines are known not to meaningfully induce anti-stalk antibodies against the highly conserved HA domain, investigators were surprised to discover that healthy middle-aged adults mounted a moderately strong antibody response to the stalk domain of HA in Flublok.7 Why?
First, the stalk domain of this highly purified HA may be more exposed than membrane-embedded HA in split-virion vaccines. Second, the recombinant HA in Flublok is produced in insect cells, which attach smaller N-linked glycans to proteins than do mammalian or avian cells. Less glycosylation of HA antigens could correlate with a broader immune response and better viral neutralization activity; thus, the lesser glycosylation of Flublok HA than split-virion HA may permit a more robust antibody response to Flublok’s HA stalk antigens.
Could Flublok Ameliorate Mismatch Due to Antigenic Drift?
The complex and cumbersome 70-year-old egg-based flu vaccine development and production cycle extends over the better part of a year (Figure 3). To ensure enough doses can be delivered ahead of the upcoming flu season starting in September/October, candidate vaccine virus strains must be decided upon at the WHO vaccine composition meeting in February. These selections follow complex analysis of isolates collected from the preceding few months by National Influenza Centers (NICs) throughout the world.
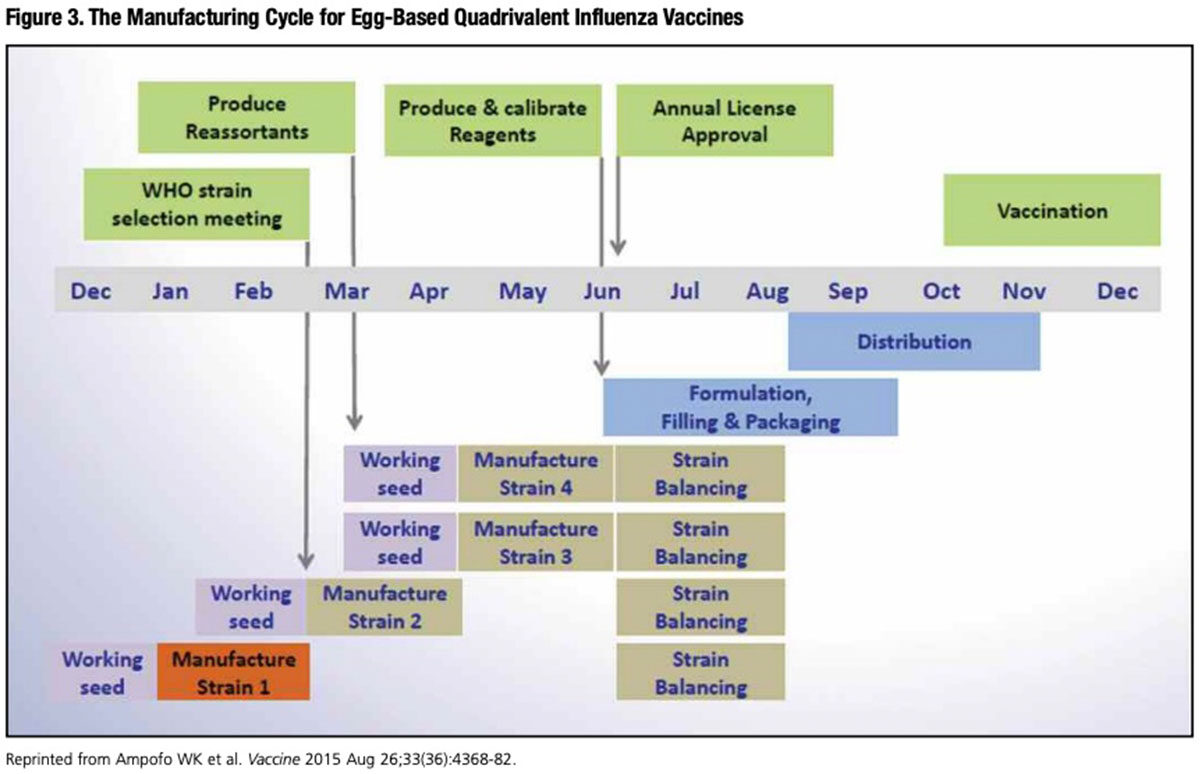
Thus, between February and the start of the influenza season, ever-mutating influenza virus strains in circulation have many months to undergo enough antigenic drift to at least partially escape vaccine-mediated neutralization.
This is exactly what occurred during the 2014-2015 flu season. After an A/H3N2 virus was selected for vaccine manufacture, a number of variant A (H3N2) viruses emerged. These late variant viruses were not well neutralized by antisera to the WHO-selected A/H3N2 vaccine virus. Unfortunately, these late-emerging A/H3N2 strains predominated during the 2014-2015 flu season, resulting in very low vaccine effectiveness.
A Centers for Disease Control and Prevention (CDC) look back at the progression of test findings is instructive. During the preceding 2013-2014 flu season during which an A/H1N1 strain predominated, CDC characterized 86 A/H3N2 viruses, all of which were vaccine-like. In February 2014, less than 1 percent of A/H3N2 viral isolates tested by CDC exhibited a reduced titer to the selected A/H3N2 vaccine virus sera. But by April, the corresponding figure for reduced titers had increased to 11 percent, then jumped to 31 percent in May.
By September 2014, 49 percent of isolates tested by CDC had reduced titers to the vaccine antisera. The result of this antigenic drift and consequent antigenic divergence between vaccine and circulating viruses: an overall vaccine effectiveness of just 19 percent for the 2014-2015 flu season. For adults aged 65 years and older, the flu-related hospitalization rate reached the highest level since record keeping started in 2005-2006.
To try to address this problem of late-emerging flu virus variants, U.S. health officials explored scenarios involving delaying the vaccine virus decision from February to mid-April, or revising virus selection to allow a changed recommendation for one vaccine virus as late as mid-June. It became apparent that, given the long timeline for egg-based vaccine production, delaying the virus strain selection beyond mid- to late-March would adversely impact vaccine availability, and thus was not feasible.8
As noted earlier, significant mutations are also generated when selected wild-type vaccine viruses — in particular the H3N2 subtype — are genetically adapted to improve their growth rate in embryonated eggs. In the process of producing these egg-adapted “high-growth reassortants” optimized for egg-based vaccine manufacture, new mutations may be introduced in the HA antigen that again can further diminish the immunogenicity of inactivated reassortant vaccine virus against the wild-type epidemic virus strain.
In contrast, Flublok is manufactured without growing influenza virus at all (Figure 4). As a result, there is no risk of introducing mutations of the kind caused by replication of the influenza virus to improve growth in eggs. Influenza viral RNA encoding each of the four full-length HA proteins (from four selected A and B influenza subtypes) is reverse transcribed to produce a cDNA that is cloned into a baculovirus expression vector. The recombinant baculovirus vector is then combined with host insect cells in large-scale bioreactors. The baculovirus-infected cells are then cultured, harvested and HA antigens are extracted, purified, blended together and filled into single-dose prefilled syringes.9 A licensed human papillomavirus vaccine (Cervarix; GSK) is manufactured using a very similar baculovirus expression system.
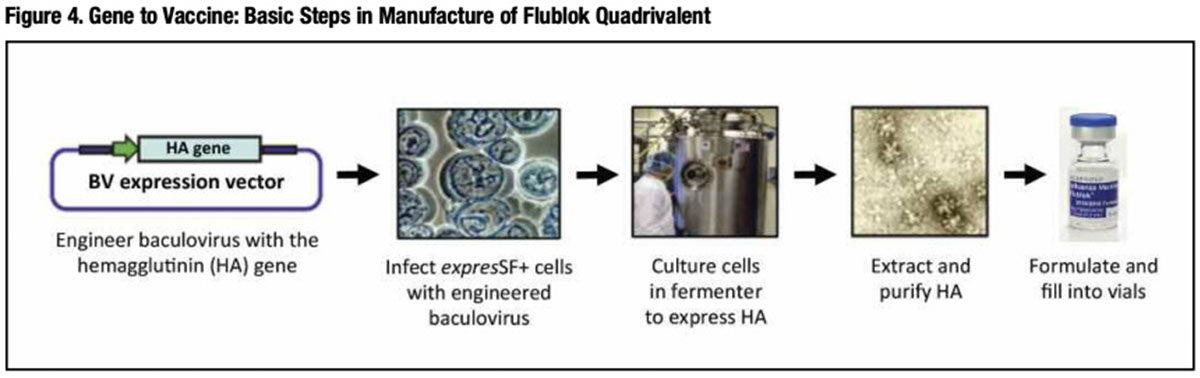
From receipt of HA genetic sequences from WHO-selected and FDA-approved vaccine virus strains, large-scale production of Flublok can be completed in about eight weeks — several months faster than egg-based IIV production. It is for this reason that development of Flublok has received critical support from the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA), which strongly values the ability of this manufacturing strategy to expedite production and distribution of vaccine in the event of a severe flu pandemic.**
Currently, WHO Collaborating Centers convene in February to analyze data from as many as 200,000 respiratory specimens supplied by NICs in more than 90 countries between the preceding September and January to select from some 5,000 unique viral strains thought most likely to be predominantly circulating when the flu season starts eight months later.10 Very shortly thereafter, FDA makes its final strain selections that are used in vaccine development by all manufacturers — including Protein Sciences.
But the short Flublok manufacturing cycle raises an intriguing “what if” scenario with a potential payoff in improved and more consistent year-to-year flu vaccine effectiveness:
Could WHO Collaborating Centers conduct a supplemental analysis of viral isolates collected several months closer to the start of flu season — perhaps as late as May or June — to select candidate strains specifically for Flublok vaccine production with potentially closer match to strains that later emerge to become epidemic?***
It seems reasonable to expect that A and B flu strains selected from viral isolates that have propagated and genetically drifted for several months after WHO-selected strains in February are apt to be a better match to circulating strains that ultimately account for the fall/winter seasonal flu epidemic. Figure 5 presents this concept in graphic form.
Putting Flublok’s Differentiating Features to the Test
The HA antigens in Flublok are not subject to reassortment-related mutations that can occur when selected vaccine strains are subjected to egg adaptation. Reduced HA glycosylation peculiar to expression in insect cells could translate into improved effectiveness through induction of neutralizing antibody to the protein’s more exposed stalk region. Flublok Quadrivalent is formulated with three times the quantity of each of the four HA antigens as standard IIV4s to optimize its immunogenicity.
Could Flublok — manufactured from the same selected vaccine viruses as egg-based vaccines or more ideally prepared using less antigenically drifted flu strains selected months later in a second round — be more effective than egg-based vaccines in reducing seasonal influenza attack rates and flu-related complications? Of course there is a way to find out: well-designed head-to-head clinical trials in elderly, nonelderly and immunocompromised adults over multiple flu seasons, variously evaluating Flublok against standard-dose, high-dose and adjuvanted IIVs.
Large-scale influenza vaccine efficacy trials of this nature are notoriously expensive to conduct, and positive outcomes are certainly not assured. But far higher are the yearly costs in influenza-related illness, lost work days, hospitalizations and deaths for the continued failure of currently licensed vaccines to more fully protect the American public from seasonal influenza.
No manufacturer is more intimately familiar with the limitations of today’s flu vaccines than vaccines giant Sanofi Pasteur, which agreed to acquire Protein Sciences in July. “As part of Sanofi Pasteur, we expect our Flublok influenza vaccine to benefit from [its] expertise in the field of influenza vaccines,” said Protein Sciences president and CEO Manon M.J. Cox, who led development of the product. Given Sanofi Pasteur’s resources and its demonstrated commitment to influenza vaccines research, the future for this first-ever recombinant influenza vaccine — and potentially millions of people who continue to be at risk for flu-related complications — is promising indeed.
*Studies conducted by Protein Sciences found there is a limit to the boost in immunogenicity that can be attained by increasing Flublok’s HA antigen content: Doses more than three-fold higher than the standard 15 μg amount in IIV do not appreciably increase anti-HA antibody titers (Treanor JJ, Schiff GM, Couch RB, et. al. J Infect Dis 2006; 193:1223–8).
** BARDA has also supported development of Flucelvax (Seqirus), an influenza vaccine that grows whole virus in mammalian (MDCK) cells rather than eggs. Similar to Flublok, the Flucelvax manufacturing cycle is much shorter than for egg-based flu vaccines.
***This supplemental vaccine virus strain selection process could apply as well to Seqirus’ Flucelvax, which also features a much shorter production cycle than egg-based vaccines.
KEITH BERMAN, MPH, MBA, is the founder of Health Research Associates, providing reimbursement consulting, business development and market research services to biopharmaceutical, blood product and medical device manufacturers and suppliers. He also serves as editor of International Blood/Plasma News, a blood products industry newsletter.
LUKE NOLL is director of vaccine sales and corporate accounts at FFF Enterprises Inc.
References
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006 Feb;24(8):1159-69.
- Diaz Granados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. New Engl J Med 2014 Aug 14;371(7):635-45.
- Cox MM, Patriarca PA, Treanor J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respir Viruses 2008 Nov;2 (6):211-9.
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. New Engl J Med 2017 Jun 22;376(25):2427-36.
- Diaz Granados CA, Saway W, Gouaux J, etal. Safety and immunogenicity of high-dose trivalent inactivated influenza vaccine in adults 50-64 years of age. Vaccine 2015;33:7188-93.
- Steens A, Waaijenborg S, Teunis PFM, et al. Age-dependent patterns of infection and severity explaining the low impact of 2009 influenza A (H1N1). Am J Epidemiol 2011;174:1307-15.
- Nachbagauer R, Choi A, Izikson R, et al. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. mBio 2016 Jan/Feb;7(1):1-10.
- World Health Organization (WHO). Improving Influenza Vaccine Virus Selection — Report of the 4th Informal Consultation. 18-20 November 2015. Accessed at apps.who.int/iris/bitstream/10665/252614/1/9789241511841-eng.pdf?ua=1.
- Holtz KM, Anderson DK, Cox MMJ. Production of a recombinant influenza vaccine using the baculovirus expression vector system. BioProcessing Journal 2003 Sept/Oct:65-73.
- Process of influenza vaccine virus selection and development. World Health Organization (WHO). Accessed 8/2/2017 at apps.who.int/gb/pip/pdf_files/Fluvaccvirusselection.pdf?ua=1.
- U.S. Centers for Disease Control and Prevention. Seasonal Influenza Vaccine Effectiveness, 2005-2017. Accessed 8/2/2017 at www.cdc.gov/flu/professionals/vaccination/effectivenessstudies.htm.