Industry News
Research, Science & Manufacturer Updates
Biosimilars Articles
The U.S. Food and Drug Administration has approved KEYTRUDA QLEX (pembrolizumab and berahyaluronidase alfa-pmph) injection for subcutaneous administration in adults across most solid tumor indications for KEYTRUDA (pembrolizumab).
Celltrion’s Avtozma (tocilizumabanoh), a biosimilar to Actemra (tocilizumab), in both intravenous (IV)and subcutaneous (SC) formulations, has been approved by the U.S. Food and Drug Administration.
Samsung Bioepis’ biologics license application for Epysqli (eculizumab-aagh) as a biosimilar to Soliris (eculizumab) has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis and atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy.
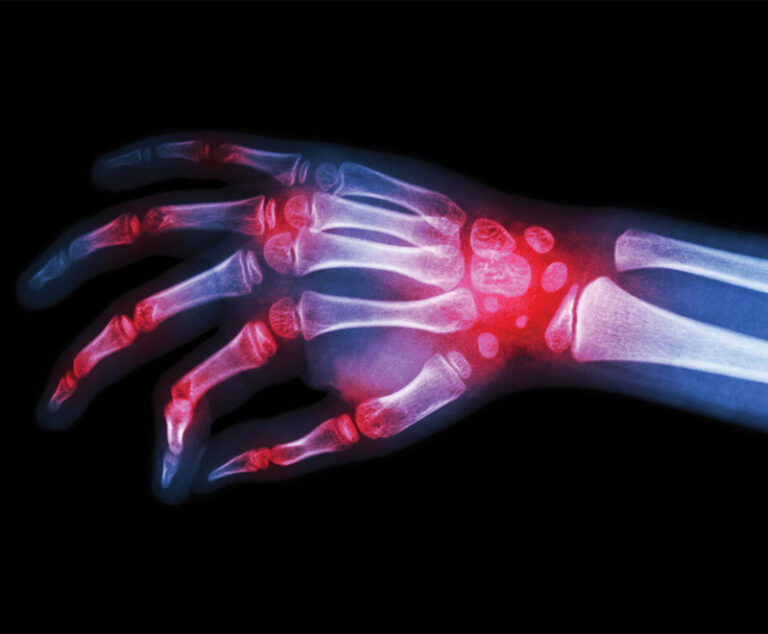
The U.S. Food and Drug Administration has approved Tyenne (tocilizumab-aazg), a biosimilar referencing tocilizumab (Actemra; Genentech), to treat multiple autoimmune diseases, including rheumatoid arthritis and juvenile idiopathic arthritis.
FDA has approved Alvotech’s Simlandi (adalimumab-ryvk) as the third interchangeable Humira biosimilar.
The U.S. Food and Drug Administration (FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara for adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy; active psoriatic arthritis; moderately to severely active Crohn's disease; and moderately to severely active ulcerative colitis.
The U.S. Food and Drug Administration has approved Samsung Bioepis; Halima (adalimumab-bwwd) for the treatment of several autoimmune disorders, including rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and Crohn's disease.
Amgen’s biosimilar to Johnson & Johnson’s rheumatoid arthritis drug, Remicade, has been approved by the U.S. Food and Drug Administration.
The U.S. Food and Drug Administration (FDA) has approved Ziextenzo (pegfilgrastim-bmez), the 24th biosimilar approval in the U.S.
Pfizer’s Nivestym (filgrastim-aafi) has been approved by FDA for all eligible indications of the reference product.
The U.S. Food and Drug Administration approved Mvasi (bevacizumab-awwb), a biosimilar to Avastin (bevacizumab), the first biosimilar drug to treat cancer.
Mylan’s Ogivri (trastuzumab-dkst) has been approved by the U.S. Food and Drug Administration as a biosimilar to Genentech’s Herceptin (trastuzumab).