Drug Pricing Reform and Cost Containment
- By Bonnie Kirschenbaum, MS, FASHP, FCSHP
With the cost of medications in hospitals, outpatient areas, physician offices and at home surging at an unprecedented rate, the emphasis is on drug pricing reform and cost management. But choosing and using expensive products wisely and judiciously is often perceived by some as limiting choice or rationing, eliciting a pushback against restrictions in product use or cost sharing.
While drug breakthroughs greatly benefit those who need them, costs can be prohibitive. Today, immunotherapies are in the limelight as wonder drugs for a number of disease states. According to the research firm GlobalData, the “global market for cancer immunotherapies alone is expected to grow more than four-fold globally to $75.8 billion by 2022 from $16.9 billion in 2015.” In addition, a news report by Reuters states that competition between next-generation cancer drugs “is not reining in prices,” with some medications costing more than $250,000 each year. Even for a patient with the highest level of insurance coverage, a modest copayment of 10 percent would translate to an annual out-of-pocket expenditure of $25,000.
Drug Pricing
Myriad factors ultimately contribute to the list price of a product. For one, the complicated and convoluted nature of providing healthcare in the U.S., with its multiple administrative and management layers, contributes to high pricing. It is often a tussle between who controls and manages the dollars spent. The words “control” and “manage” in any capacity translate into a cost that is added on to the product itself before it ultimately reaches the patient.
Obviously, a number of basics contribute to the baseline cost of a drug. Years of expensive research and discovery, development and the arduous and lengthy process of clinical studies seeking to meet the requirements of U.S. Food and Drug Administration approval consume vast amounts of capital. Following that comes manufacturing the product in a manner that can be brought to scale in a rigidly controlled environment.
Simultaneously, other teams are tasked with pricing the product, creating marketing strategies and determining distribution channels. Data analytics plays a major role in determining the value of a new drug and in setting price. So does who the decision maker is. Currently, the key decision maker is moving away from physician preference in favor of payers (insurance companies), employers and patients. For instance, direct-to-consumer advertising of pharmaceuticals that costs in the billions of dollars is built into the price of the product, along with all other marketing expenses. In contrast with other countries, the U.S. is almost unique in allowing this marketing strategy.
The newest strategy is to set lower prices for new products, especially if there are no direct competitors or the new product meets an unmet need. However, this may not bode well for the many new drugs that don’t target a unique niche or an unmet medical need. Two good examples of this occurred in late March, when pharmaceutical companies set lower-than-normal prices for two newly approved drugs, an eczema drug from Sanofi and Regeneron, and a multiple sclerosis drug from Roche. The eczema drug will cost $37,000 a year, compared to the $50,000-a-year price tags for similar, yet older treatments. The multiple sclerosis treatment is proposed to cost $65,000, which is a 25 percent decrease from the price of a competing treatment approved 15 years ago.
However, simply setting lower prices is not the end of this complex journey, and in the current environment, many other factors weigh into the ultimate price decision.
One factor is competition, which is needed to offer discounts to gain ground. But, there is a cost to discounting that is built into the price of the drug. The logic is if there is less competition and less need to offer discounts, the price can be lowered at no financial loss to the manufacturer.
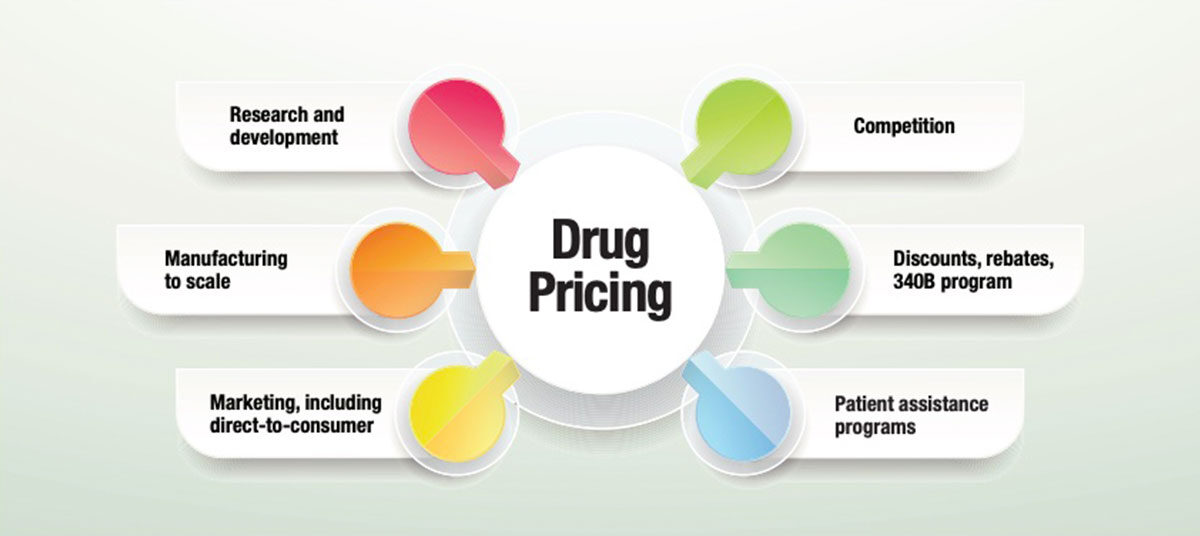
Another factor is rebates that are paid for a variety of prenegotiated terms. These range from Medicaid rebates for outpatient prescription drugs, to pharmacy benefit manager (PBM) rebates for favoring product selection and group purchasing organization rebates for performance. On a smaller scale, a rebate may be for a therapeutic interchange program. The list is endless, but the concept is the same. The cost of a rebate is built into the price of the product. Where the dollars from the rebate go and who benefits from them is another issue.
Last issue, this column covered the 340B program and complexities of offering discounted prices on outpatient drugs to qualified entities that assume significant responsibilities for appropriate use of the program and appropriate use of the savings they receive. The cost of 340B discounts is built into the drug price formula as well. But it’s not only the cost of the discounts, it’s also the tremendous costs of administering the program that trickle into the pricing formula. Aside from military and VA price controls, the 340B program remains the only price-controlled program in the U.S. Those that qualify greatly benefit, but those that offer similar services but don’t qualify are paying a nondiscounted price that has the cost of other discounts and administrative costs built in.
Patient assistance programs offered by the pharmaceutical industry are a welcome lifeline to those who qualify for the zero-priced or nominally priced products, but once again, the cost of the drugs and administration of the program both are built into the formula.
Cost Containment
When faced with growing expenses that loom larger than the budget to pay for them, cost containment and cost management quickly gain traction with both healthcare providers and payers. Once again, it’s vital that the administrative costs don’t overwhelm the savings.
Among others, some popular options include a closed formulary, prior authorizations from insurers, local and national coverage determinations from the Centers for Medicare and Medicaid Services, PBM management strategies, specialty pharmacies, closed distribution systems and one of the newer concepts, pay for performance. Although some may see these as odious, there is no question there is a vital need to choose and use expensive products wisely and judiciously. Administrative simplification would go a long way too.