Coming Soon: A Highly Effective New Vaccine to Prevent Shingles
- By Keith Berman, MPH, MBA
IN ITS BEST-known clinical manifestation as chickenpox, primary infection with the varicella zoster virus (VZV) has left generations of children with lasting memories of itchy, uncomfortable misery. But this common virus does something quite extraordinary: In everyone it infects, it manages to evade complete immune clearance by remaining latent in the dorsal root or cranial nerve ganglia. There, VZV waits — for decades — until weakened or suppressed host immunity allows it to reactivate.
The second incarnation of VZV as herpes zoster, or shingles, starts with localized unilateral burning or tingling skin pain limited to a small area, followed by a rash that turns into fluid-filled blisters. For some individuals, the course is relatively mild, while others suffer intense pain with the gentlest touch or breeze on the skin.
The risk of shingles rises steeply after age 50 years, as innate immunity that has kept VZV in its latent state begins to decline. Due to natural immunosenescence, an 80-year-old faces roughly 10 times the risk of developing shingles than a 50-year-old. Immunosuppressive drug therapy or immunodeficiency disorders also predispose individuals to higher risk of shingles.
Most cases of shingles resolve within three to five weeks. But it can lead to an array of neurological, ocular, auditory, infectious and other complications.1 By far, the most frequent of these is postherpetic neuralgia (PHN), defined as pain lasting at least 90 days following resolution of the rash. As people get older, they are more likely to develop PHN. This ongoing severe pain, which often lasts for many months or years, may interfere with daily activities like dressing, cooking and eating, and can lead to insomnia, chronic anxiety, depression and weight loss.
Approximately one million cases of shingles are reported annually in the U.S., and we all have about a one-in-three lifetime risk, according to Centers for Disease Control and Prevention estimates. Half of individuals over age 85 years are likely to develop shingles.2 The need for a vaccine that can provide protective immunity against shingles and PHN in older at-risk adults is clear.
ZOSTAVAX: The First Preventive Vaccine for Shingles
Merck responded to this need by developing ZOSTAVAX, a live-attenuated zoster vaccine licensed by the U.S. Food and Drug Administration (FDA) in 2006. It was approved following review of the company’s Shingles Prevention Study (SDS) trial, which randomized more than 38,000 subjects aged 60 years and older to receive a single dose of ZOSTAVAX or placebo. They were followed for a median of just over three years, and confirmed shingles cases were evaluated for three age strata: 60 to 69 years, 70 to 79 years and 80 years and older. A separate trial randomized more than 22,000 subjects between ages 50 and 59 years, and followed them for a median of 1.3 years.
The overall effectiveness of ZOSTAVAX to prevent shingles disease was 51 percent in subjects 60 years of age and older. But the vaccine’s efficacy differed widely by age group (Figure 1). The SDS study documented 70 percent and 64 percent nominal reductions in the incidence rate of shingles in subjects aged 50 to 59 years and 60 to 69 years, respectively. But in subjects aged 70 to 79 years, the vaccine reduced risk of shingles by only 41 percent, and in those aged 80 years and older, it offered minimal or no protection (18 percent; statistically nonsignificant).
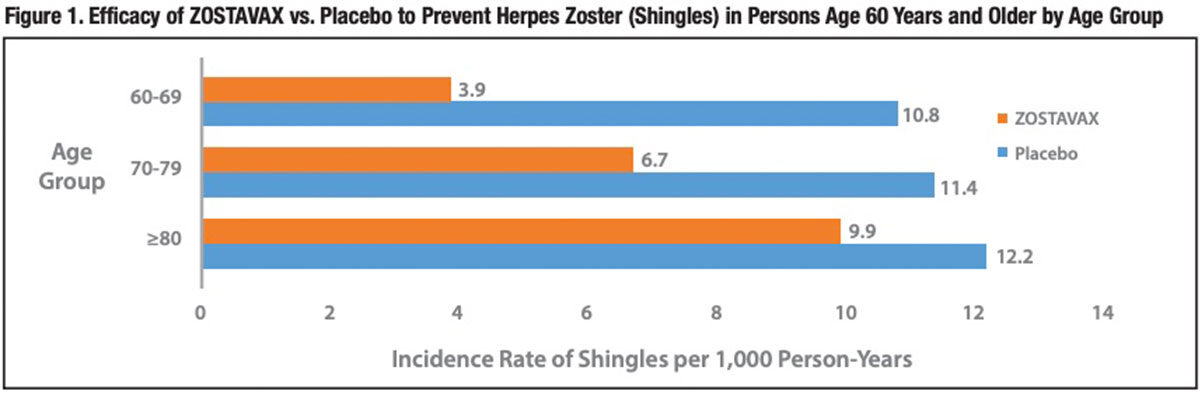
The 51 percent overall reduction in incidence of shingles was actually exceeded by a nearly 67 percent lower risk of PHN.3 This reflects the finding that vaccinated subjects diagnosed with shingles were nearly 40 percent less likely to progress to PHN than were placebo control subjects who developed shingles (8.6 percent versus 12.5 percent; 95 percent confidence interval [CI], 7 percent to 59 percent). But, clearly, the primary benefit of ZOSTAVAX was its ability to prevent many cases of herpes zoster.
While ZOSTAVAX importantly reduces the risk of shingles in adults older than 50 years, its efficacy is clearly more limited for people in their 70s, then precipitously declines for those in their 80s — the two age groups with the highest disease risk and disease burden. And because it is a live vaccine, ZOSTAVAX is contraindicated for immunosuppressed or immunodeficient individuals.
Two recent observational studies additionally found the vaccine’s effectiveness markedly declines over time; by the ninth postvaccination year, it has no significant protective effect against shingles.4,5 Thus vaccination in one’s 50s, for example, provides peak protection at a time when shingles is much less likely to occur. This limitation may be addressable, however: A recent Merck-sponsored study found that administration of a booster dose of ZOSTAVAX 10 or more years after initial vaccination was well-tolerated and elicited a humoral and cellular immune response of similar magnitude to individuals receiving their first dose.6
SHINGRIX: Evidence of Superior Efficacy
More than 15 years ago, GlaxoSmithKline (GSK) initiated development of its own herpes zoster vaccine with a number of aspirations in mind:
- High vaccine efficacy in persons 50 years and older, and in particular more elderly persons (70 years and older) at highest risk for shingles and PHN
- Safety and efficacy in all persons at increased risk for shingles, including immunocompromised persons
- Prolonged duration of protection
- Ease of manufacture and reliability of supply
To try to fulfill this ambitious set of aspirations, GSK designed a nonlive VZV subunit vaccine dubbed HZ/su, with the proposed brand name SHINGRIX.* It combines a viral antigen (recombinant glycoprotein E) with a novel adjuvant called AS01B (Figure 2). Glycoprotein E is the most abundant protein found on the envelope of VZV. AS01B is a liposome-based adjuvant system that contains two immunostimulants: 3-O-desacyl-4’- monophosphoryl lipid A (MPL), which enhances cellular and humoral immunity, and the saponin QS-21, which is known to induce Th1 cell-mediated immunity and cytotoxic T-lymphocyte activity. MPL and QS-21 act synergistically to generate an enhanced proinflammatory response to copresented antigen; this recruitment of innate immunity results in a more rapid, stronger and longer-lasting immune response.7
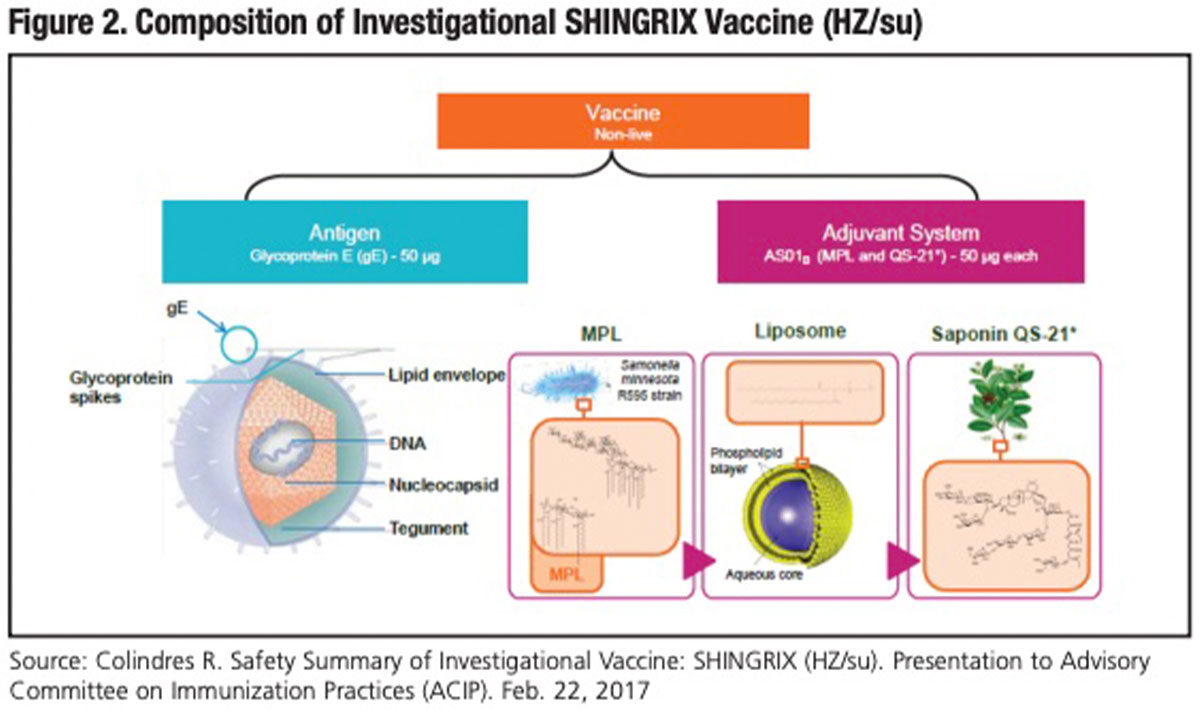
Early safety and immunogenicity studies guided both the most optimal combination of adjuvant and antigen (50 μg each of glycoprotein E, MPL and QS-21) and a regimen of two intramuscular doses administered two months apart.
Two randomized, placebo-controlled Phase III clinical trials were conducted to assess the efficacy and safety of SHINGRIX. The first, ZOE-50, evaluated the two-dose regimen in 15,411 participants 50 years and older, stratified according to age group (50 to 59, 60 to 69 and 70 and older). After a mean follow-up of 3.2 years, just six participants in the vaccine group had confirmed shingles, compared with 210 in the placebo group. This translated into an overall vaccine efficacy of 97.2 percent (95 percent CI, 93.7 percent to 99.0 percent; P<0.001). Even more remarkable was the finding that vaccine efficacy was similarly high across the three successively older age groups: 96.6 percent, 97.4 percent and 97.9 percent.8
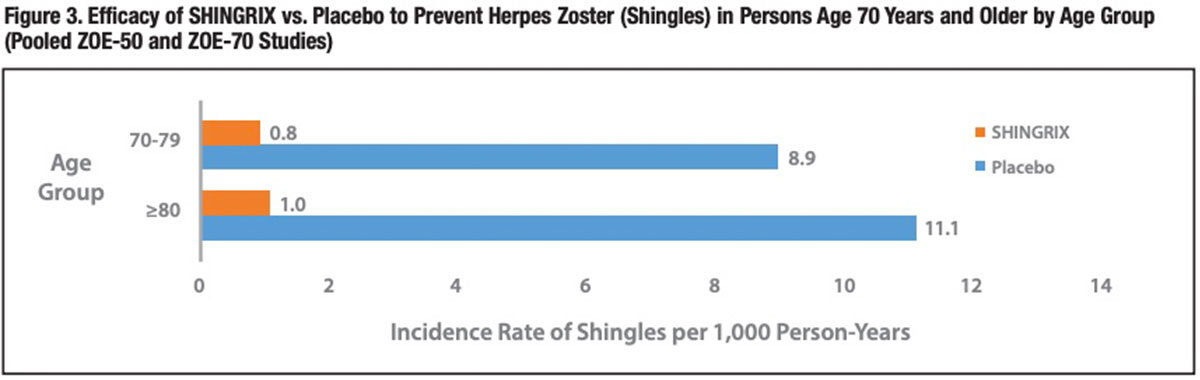
A second trial conducted in an identical manner (ZOE-70) narrowed the focus to 13,900 adults 70 years of age and older, who are at the highest risk of shingles and severe PHN. During a somewhat longer mean follow-up period of 3.7 years following completion of two doses of SHINGRIX or saline placebo injections, shingles occurred in 23 vaccine recipients and in 223 placebo recipients (0.9 versus 9.2 per 1,000 person-years), translating into a vaccine efficacy of 89.8 percent (95 percent CI, 84.2 percent to 93.7 percent; P<0.001). There was no significant difference in shingles risk between the 70- to-79-years and 80-years-and-older cohorts, affirming the ZOE-50 findings. 9 No important vaccine safety concerns were identified in either the ZOE-50 or ZOE-70 trial.
A pooled analysis of nearly 16,700 participants in the ZOE-50 and ZOE-70 trials documented approximately 90 percent vaccine efficacy for both the 70-to-79-years and 80-years-and-older cohorts (Figure 3). For the overall 70- years-and-older cohort, the incidence rate of PHN was 0.1 case per 1,000 personyears for the SHINGRIX group and 1.2 cases per 1,000 person-years for the placebo group — a similar 89 percent risk reduction (P<0.001).
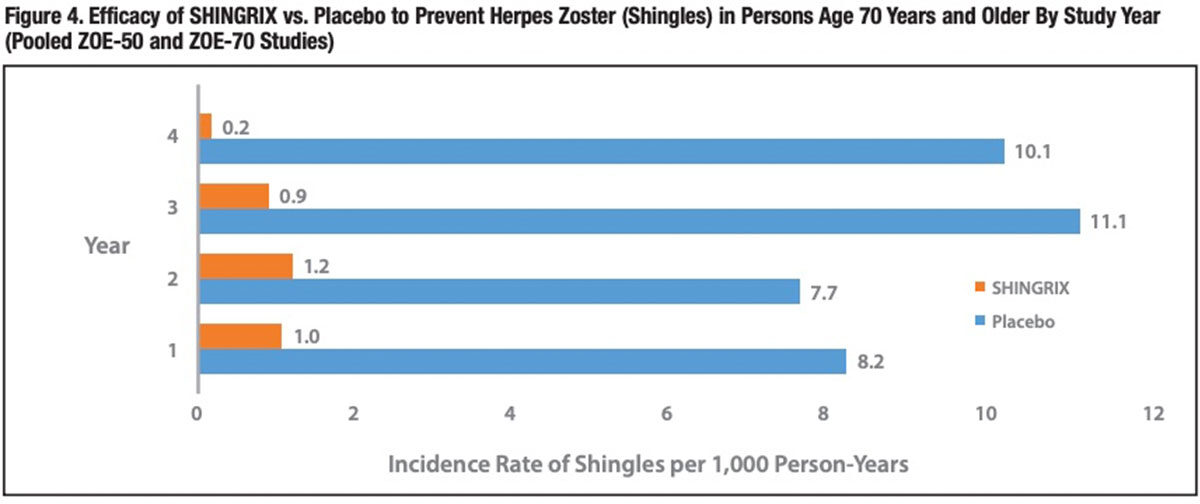
The pooled analysis also revealed that SHINGRIX’ very high protective efficacy persisted over the entire four-year follow-up period (Figure 4). GSK is conducting a ZOE-50 and ZOE-70 extension study to characterize the efficacy, safety and immunogenicity persistence of SHINGRIX up to 10 years postvaccination.
Although the immunological basis for this strong and lasting protective effect is not known, the investigators postulate that it is partly attributable to the demonstrated ability of SHINGRIX to induce strong glycoprotein E-specific CD4+ T-cell responses that are preserved with age.10 “The robustness of the immune responses to glycoprotein E are attributable to the action of the AS01B adjuvant system,” they conclude, “which has also been shown to enhance CD4+ T-cell and humoral immune responses to subunit antigens from other pathogens.”7 These include antigens isolated from HIV, mycobacterium tuberculosis, hepatitis B virus and human papillomavirus.11
More SHINGRIX Studies in the Pipeline Last October, GSK submitted a biologics license application with FDA seeking approval to market SHINGRIX for the prevention of herpes zoster/shingles in people 50 years and older. A decision by FDA is anticipated by late October. Meanwhile, GSK has clinical trials underway to evaluate SHINGRIX in immunosuppressed and certain immunodeficient persons at increased risk of shingles, including:
- Solid and hematological cancer patients
- Hematopoietic stem cell and renal transplant recipients
- Persons infected with HIV
A clinical study is also underway to evaluate revaccination in subjects who have previously been vaccinated against shingles with the currently available live attenuated vaccine.
As effective as the two-dose SHINGRIX vaccination regimen appears, some individuals still experience breakthrough shingles and PHN. Not content with this, GSK initiated a multinational clinical study last year to evaluate the safety, immunogenicity and efficacy of one or two additional doses in persons 50 years and older. This study will enroll more than 7,700 subjects and is projected to be completed in 2023.
In a study of the costs of medically managing shingles and PHN published earlier this year, Canadian investigators concluded that “the likely future of herpes zoster burden is one of rising costs, primarily driven by the demographic shifts of an increasing and aging population.” The new subunit vaccine from GSK promises an entirely different future, one largely absent of the profound suffering or the economic burden of this human scourge.
* Subject to approval by relevant regulatory review bodies.
KEITH BERMAN, MPH, MBA, is the founder of Health Research Associates, providing reimbursement consulting, business development and market research services to biopharmaceutical, blood product and medical device manufacturers and suppliers. Since 1989, he has also served as editor of International Blood/Plasma News, a blood products industry newsletter.
LUKE NOLL is director of vaccine sales and corporate accounts at FFF Enterprises Inc.
References
- Cohen JI. Herpes zoster. New Engl J Med 2013 Jul 18;369(3):255-63.
- U.S. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th ed., 2015.
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. New Engl J Med 2005 Jun 2;352(22):2271-84.
- Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015 Mar 1;60(6):900-9.
- Tseng HF, Harpaz R, Luo Y, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis 2016 Jun 15;213(12):1872-5.
- Levin MJ, Schmader KE, Pang L,etal. Cellular and humoral responses to a second dose of herpes zoster vaccine administered 10 years after the first dose among older adults. J Infect Dis 2016 Jan 1;213(1):14-22.
- Dendouga N, Fochesato M, Lockman L, et al. Cell-mediated immune response to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 2012 Apr 26;30(20):3126-35.
- Lal H, Cunningham AL, Goxeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. New Engl J Med 372(22):2087-96.
- Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. New Engl J Med 2016 Sep 15;375(11):1019-32.
- Chlibek R, Pauksens K, Rombo L, et al. Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine 2016;34:863-8.
- Colindres R. Safety Summary of Investigational Vaccine: SHINGRIX (HZ/su). Presentation to Advisory Committee on Immunization Practices (ACIP), Feb. 22, 2017. Accessed 5/5/2017 at www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/zoster-02-gsk.pdf.