Going Up: What’s Behind the Growth in IVIG Demand
- By Keith Berman, MPH, MBA
“There is still much to be learned about immunoglobulin therapy, and many more clinical applications may yet emerge.”1
— Stephen Jolles, University Hospital of Wales
Srini Kaveri, French Institute of Health and Medical Research
Jordan Orange, University of Pennsylvania School of Medicine
LAUNCHED IN EUROPE in the late 1970s and here in the U.S. in 1981, the first intravenous immune globulin (IVIG) products transformed the lives of patients diagnosed with primary immunodeficiency (PI) disorders, freeing them from frequent painful — and usually suboptimal — doses of older intramuscular preparations. Monthly infusions of IVIG dramatically reduced severe recurring bacterial infections, hospitalizations and lost school or work days for these individuals.
But several early IVIG products were also introduced with a second labeled indication: treatment of immune thrombocytopenic purpura (ITP). Totally unexpected, it resulted from a chance observation by Swiss investigators: When IVIG was given to a 12-year-old boy with hypogammaglobulinemia secondary to immunosuppressive therapy for severe chronic ITP, his platelet counts dramatically increased.2,3 The Swiss and others who confirmed this finding in other ITP patients could only speculate how these IgG antibody solutions, purified from pooled plasma from thousands of healthy donors, somehow managed to block autoimmune platelet destruction. But it really didn’t matter: IVIG preparations worked, they were well-tolerated, and they had fewer side effects compared to standard treatment options.
By 1990, U.S. sales of IVIG totaled about 6.6 million grams, according to Patrick Robert, whose Connecticut-based Marketing Research Bureau tracks and reports worldwide sales of human plasma-based therapeutics. Twenty-five years later, 2015 sales of IVIG and newer subcutaneous immune globulin (SCIG) products approved for PI had increased 10-fold to 67.3 million grams (Figure 1).
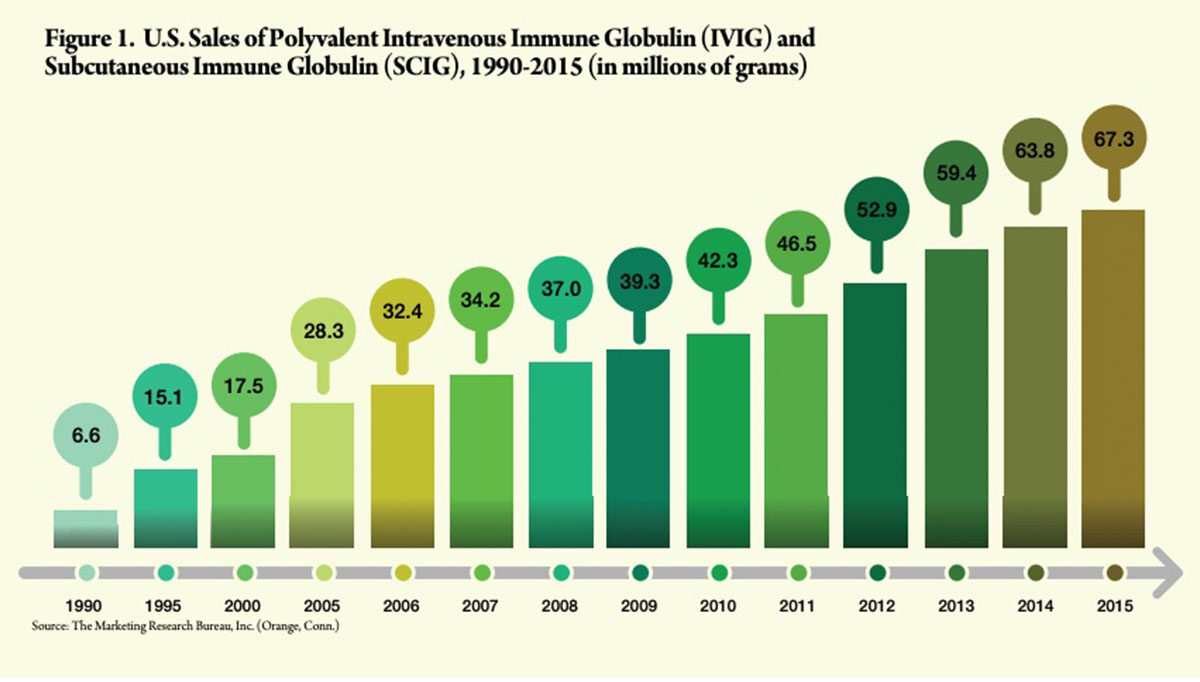
But remarkably, more than three decades after its introduction, the U.S. market for IVIG continues to grow well over 5 percent annually. While PI and ITP generated most early IVIG prescribing activity, and remain important today, these two indications now account for only about one-third of total usage.4 Clearly something else has and continues to propel this unparalleled record of growth in market demand.
Much Research, Many Disease Targets
Beginning with that chance ITP discovery in 1980, the “something else” has been an intense and unabated interest in the potential clinical utility of IVIG for a diverse array of difficult-to-treat immune-mediated conditions. “A striking anti-inflammatory effect of IVIG has been observed” in a number of autoimmune disorders, panelists agreed at a National Institutes of Health Consensus Development Conference in 1990, citing in particular its ability to reduce fever, neutrophil counts and acute phase reactants in Kawasaki disease.5 “There may be multiple mechanisms of IVIG action,” they suggested. Further investigations have subsequently identified a spectrum of immunoregulatory actions associated with these polyvalent IgG products that could account for their potent therapeutic effects in certain autoimmune disorders.6,7
difficult-to-treat immune-mediated conditions. “A striking anti-inflammatory effect of IVIG has been observed” in a number of autoimmune disorders, panelists agreed at a National Institutes of Health Consensus Development Conference in 1990, citing in particular its ability to reduce fever, neutrophil counts and acute phase reactants in Kawasaki disease.5 “There may be multiple mechanisms of IVIG action,” they suggested. Further investigations have subsequently identified a spectrum of immunoregulatory actions associated with these polyvalent IgG products that could account for their potent therapeutic effects in certain autoimmune disorders.6,7
A 1992 review article in The New England Journal of Medicine cited more than 35 diseases “thought to be produced by immunopathology” for which IVIG had been reported to be beneficial. Additional clinical studies have subsequently affirmed the efficacy of IVIG for some of them — Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy and Kawasaki disease, for instance — while failing to conclusively document its value in others such as Sjögren’s syndrome and systemic lupus erythematosus. Since 1992, literally thousands of randomized clinical trials (RCTs) and case reports have characterized the therapeutic effects of IVIG in many dozens of immune-mediated disorders. Yet to this day, little is known about how IVIG actually works across a wide range of autoimmune hematological disorders, neuropathies, myopathies, neuromuscular junction disorders, mucocutaneous blistering diseases and other immune-mediated disorders for which it is routinely prescribed (Table 1).
The overall magnitude of IVIG demand is a function of that extraordinary diversity of diseases for which IVIG has been shown to be effective. But why IVIG prescriptions continue to grow — rather than having peaked and leveled off years ago like most drugs — has everything to do with what isn’t known about how it works or the diseases it treats.
Unanswered Questions Limit Prescribing
Despite years of investigation, there are many immune-mediated disorders for which the effectiveness of IVIG remains uncertain or controversial. For other disorders, sufficient case reports and small case series have documented therapeutic benefit of IVIG in some patients, but critical questions remain:
Are there definable parameters to help target IVIG therapy to potential responders, and minimize futile treatment? How much IVIG is required to achieve a therapeutic effect? How often should IVIG be administered? Depending on the condition, any or some combination of the following factors may be impeding efforts to answer these questions:
- A poor understanding of the pathogenesis of most immune-mediated disorders for which IVIG has been shown to be beneficial in at least some patients.
- The existence of characterized and possibly unknown disease variants, where IVIG may be effective in some but not others.
- A common lack of validated laboratory or clinical prognostic parameters to enable physicians to target IVIG therapy to the subset of patients that is likelier to respond.
- A paucity of validated laboratory parameters that may be used to guide IVIG dosage and dosing frequency.
- Typically very low disease prevalence or rarity, making the conduct of adequate dose-ranging and efficacy trials difficult or impractical.
The history of investigation of IVIG for multiple sclerosis (MS) offers an instructive example of the long and sometimes tortuous path of scientific discovery, clinical consensus and insurance coverage that ultimately reflects that consensus:
1992: IVIG dosed at 2 g/kg followed by bimonthly booster doses sharply reduces annual exacerbation rate in 10 patients with relapsing-remitting MS (RRMS)8
1997: IVIG is effective in monthly doses up to 2 g/kg in a randomized, placebo-controlled trial in 148 patients with RRMS9
2004: No clinical benefit of IVIG (1 g/kg/month) in a placebo-controlled RCT in 318 European patients with secondary progressive MS10
2005: An observational study of high-dose IVIG in 308 patients with RRMS documents a 69 percent reduction in the mean annualized relapse rate, similar to results of several previous RCTs evaluating IVIG for RRMS11
When administered at higher doses,12 IVIG has consistently been shown to reduce the mean exacerbation rate in RRMS patients. Several other studies have confirmed, on the other hand, that IVIG has little or no therapeutic benefit for chronic progressive forms of the disease. More than a decade after the first positive studies, most health insurers now cover IVIG for RRMS as second-line therapy subject to certain criteria, typically including failure of standard MS drug treatments.13,14,15
including failure of standard MS drug treatments.13,14,15 Unfortunately the available clinical data still don’t fully elucidate when to treat, how much to administer or which patients are likely responders or nonresponders. “IVIG therapy in RRMS has met with uncertainties that might be attributed to small patient cohorts, heterogeneity in the patients, dose of IVIG, or the duration and window of treatment,” noted authors of a recent commentary.16 And with these uncertainties about when and how to use IVIG naturally comes a hesitancy on the part of physicians to prescribe it, and insurers to agree to cover it.
But very recently, a team of Austrian and German collaborators reported findings from a forward-thinking study whose entire purpose was to determine if genotyping and functional immune parameters can be used to predict subpopulations of RRMS patients who will benefit from IVIG from those who will not. Applying a scoring system to these biomarkers, the investigators found that IVIG responders scored either 0 or 1 (indicative of milder disease), while nonresponders scored between 7 and 9.17
A larger multicenter, controlled, randomized Phase IIIb trial is now in progress by the same investigators. Should this and similar studies confirm the ability to use biomarkers to prospectively predict the subpopulation of RRMS patients who will benefit from treatment, we could see much wider utilization of IVIG as a part of the standard therapeutic armamentarium to treat RRMS.
More Research, Better Clinical Guidance Ahead
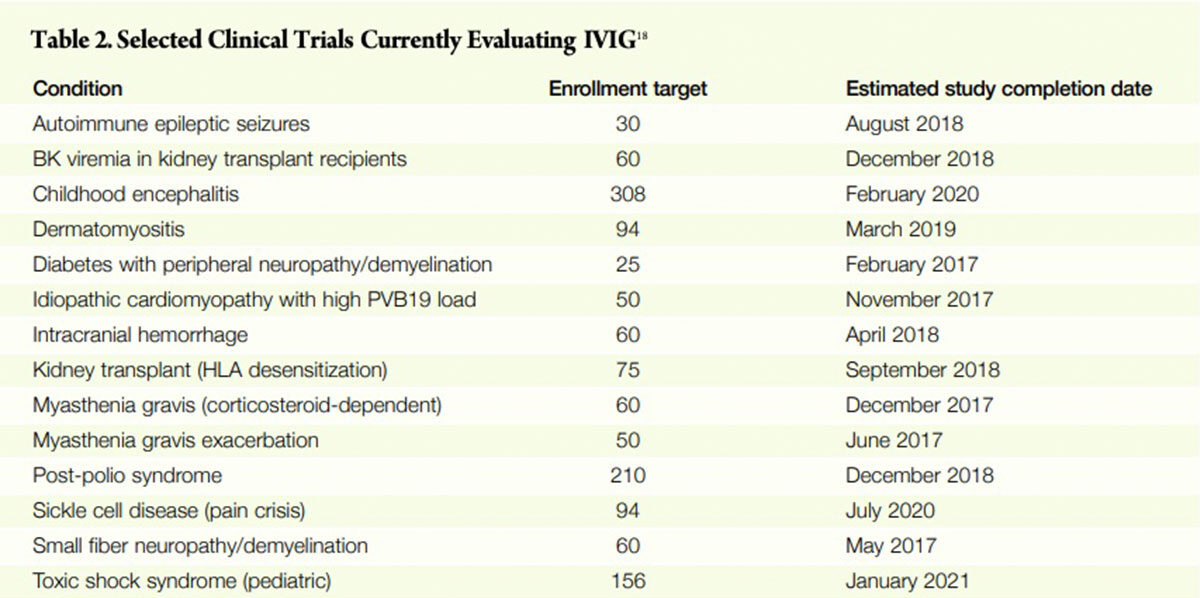
There are numerous clinical studies in progress (Table 2) addressing immune-mediated disorders, whose aim is to produce information both to establish efficacy and to help guide clinician decision-making about when, how much and for whom IVIG therapy is appropriate. Still other studies will be organized in the future. One by one as they are completed in coming years, the efficacy and safety of IVIG will be confirmed for more disease states. Equally important, better information will become available to guide appropriate patient selection, timing of therapy and dosing of clinical indications for which IVIG is known to work in some patients but not others.
And therein lies the answer to the mystery of why demand for IVIG continues to increase after all these years. IVIG is arguably the most complex therapeutic agent of any kind in existence. It is literally a concentrate of the largest component of the humoral immune system, whose mechanisms of action for a host of immune-mediated disorders remain poorly understood. More knowledge gleaned from well-designed clinical studies about the appropriate use of IVIG further empowers clinicians to use it and insurers to cover and pay for it.
But the challenge of organizing, funding and conducting trials in uncommon diseases that tend to be heterogeneous and little understood, with a product whose mode of action is also little understood, means that answers will continue to come slowly. Which all but assures that, for many years ahead, this slow stream of discovery will continue to light the path for IVIG to help yet more patients in need.
References
- Jolles S, Kaveri SV, Orange J. Intravenous immunoglobulins. Current understanding and future directions. Clin Exp Immunol 2009 Dec; 158 Suppl 1:68-70.
- Imbach P, Barandun S, d’Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981 Jun 6;1(8232):1228-31.
- Immunotherapy with Intravenous Immunoglobulins(P. Imbach,ed.). 1991: Academic Press Inc.
- The Marketing Research Bureau, Inc. A Forecast of the Polyvalent Immune Globulin (IVIG/SCIG) Market in the United States from 2012-2017. November 2011.
- Intravenous Immunoglobulin: Prevention and Treatment of Disease. National Institutes of Health Consensus Development Conference Statement, May 21-23, 1990. Accessed at consensus.nih.gov/1990/1990IntravenousImmunoglobulin080html.htm.
- Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 2004 May 19; 291(19):2367-75.
- Knezevic-Maramica I and Kruskall MS. Intravenous immune globulins: an update for clinicians. Transfusion 2003 Oct; 43:1460-80.
- Achiron A, Pras E, Gilad R, et al. Open controlled therapeutic trial of intravenous immune globulin in relapsing remitting multiple sclerosis. Arch Neurol 1992 Dec;49(12):1233-6.
- Fazekas F, Deisenhammer F, Strasser-Fuchs S, et al. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis. Austrian Immunoglobulin in Multiple Sclerosis Study Group. Lancet 1997 Mar 1;349(9052):589-93.
- Hommes OR, Sorensen PS, Fazekas F, et al. Intravenous immunoglobulin in secondary progressive multiple sclerosis: randomised placebo-controlled trial. Lancet 2004 Sep 25;364(9440):1149-56.
- Haas J, Maas-Enriquez M, Hartung HP. Intravenous immunoglobulins in the treatment of relapsing remitting multiple sclerosis —results of a retrospective multicenter observational study over five years. Multiple Sclerosis 2005 Oct;11(5):562-7.
- A randomized, placebo-controlled trial evaluating very low IVIG doses ranging from 0.2 to 0.4 g/kg every four weeks failed to substantiate a beneficial effect in 127 patients with RRMS (Fazekas F et al. Intravenous immunoglobulin in relapsing-remitting multiple sclerosis: a dose-finding trial.) NeurologyJul 22;71(4):265-71).
- CIGNADrug and Biologic Coverage Policy (Eff. 10/15/2016). Immune Globulin Intravenous(Human). Accessed at cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/pharmacy/ph_5026_coveragepositioncriteria_Immune_Globulin_Intravenous_IGIV.pdf.
- Blue Cross Blue Shield of Massachusetts Pharmacy Medical Policy. Intravenous immunoglobulin. Accessed at www.bluecrossma.com/common/en_US/medical_policies/310%20Intravenous%20Immunoglobulin%20prn.pdf.
- United Healthcare Drug Policy (Eff. 10/15/2016). Immune Globulin (IVIGand SCIG). Accessed at www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/ Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Drug%20Policies/IVIG_policy.pdf.
- Bayry J, Hartug HP, Kaveri SV. IVIg for relapsing-remitting multiple sclerosis: promises and uncertainties. Trends Pharmacol Sci 2015 Jul;36(7):419-21.
- Berger T, Jacobi C, Haas J, et al. Predicting therapeutic efficacy of intravenous immunoglobulin (IVIG)in individual patients with relapsing remitting multiple sclerosis (RRMS) by functional genomics. J Neuroimmunol 2014 Dec 15;277(1-2):145-52. 18. Accessed 10/24/2016 at clinicaltrials.gov.