Myths and Facts: Hepatitis B
HBV infection is still widely prevalent globally, but eradication could be achieved through efforts to raise awareness and find cures for the disease.
- By Ronale Tucker Rhodes, MS
IN 2016, MORE than 650 patients at a hospital in Melbourne, Australia, were believed to be at risk of contracting hepatitis B after coming into direct contact with an infected healthcare worker. Letters mailed by the health department to notify the patients recommended a blood test to determine if they had been infected. Newspaper reports about the risk of contracting the “deadly” virus created a scare, with one woman reportedly saying “she had been crying and shaking ever since she received the health department letter.”1 Scares have also occurred in the U.S., including one in 2009 when five patients tested positive after visiting the same doctor’s office in New Jersey,2 and another in 2013, when 420 patients were notified by the Tulsa (Okla.) Health Department that they had potentially been exposed to the virus after being treated at a dentist’s office where health investigators found sterilization, staffing and other infractions.3
Hepatitis B is but one of the many strains of hepatitis, ranging from A to G, with types A, B and C the most common. While hepatitis has been around for centuries, it wasn’t until the 1940s when doctors discovered the virus responsible for it.4 Hepatitis B was officially recognized in 1967 when Baruch Blumberg, MD, and his team, while studying hemophiliac patients who had received multiple blood transfusions, identified an unusual antigen from a blood sample of an Australian Aborigine. They later determined that antigen was the cause of hepatitis B, a discovery for which Dr. Blumberg was awarded the Nobel Prize for Medicine in 1976. Just two years later, he and his colleague, Irving Millman, MD, invented the hepatitis B vaccine and the diagnostic test for hepatitis B.5
Hepatitis B virus (HBV) infects the liver and can cause liver inflammation called “hepatitis.”6 It is estimated to have infected two billion people throughout the world, 400 million of whom have chronic hepatitis B, making it one of the most common human pathogens.7 In 2014 in the U.S., there were an estimated 19,200 new infections, with an overall incident rate of 0.9 cases per 100,000 people, and an estimated 850,000 to 2.2 million total cases (depending on the study) of chronic HBV infection.8,9 Hepatitis B is widespread and can be deadly, which makes the facts surrounding the disease paramount to reducing its spread.
Separating Myth from Fact
Myth: Hepatitis B is a rare disease.
Fact: Hepatitis B is one of the most common infectious diseases in the world, having infected more than one-third of the global population.10
Myth: Only people who are suspected of being exposed to HBV should be tested.
Fact: Anyone is capable of contracting hepatitis B. People who are suspected of being exposed should definitely be tested. The Centers for Disease Control and Prevention (CDC) specifically recommends the following individuals be tested even if it is not suspected they have been exposed to HBV: all pregnant women; persons born in regions with intermediate or high rates of hepatitis B; U.S.-born persons not vaccinated as infants whose parents were born in regions with high rates of hepatitis B; men who have sex with men; injection drug users; patients with elevated liver enzymes of unknown etiology; hemodialysis patients; persons needing immunosuppressive or cytotoxic therapy; HIV-infected persons; and donors of blood, plasma, organs, tissues or semen.8
The basic test for HBV infection is called the Hepatitis B Core IgM Antibody test, which will indicate the serological markers (Table 1) that show whether an individual is immune to, susceptible to or infected with HBV.11
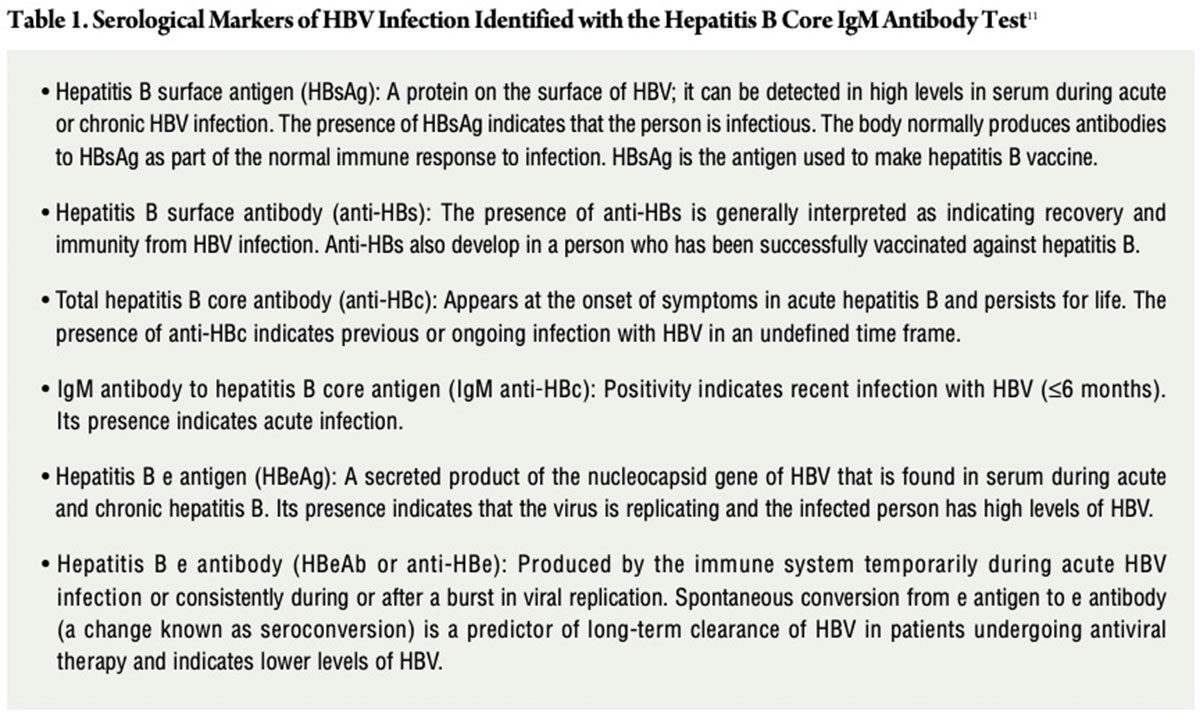
Myth: The incidence of HBV infections has been increasing.
Fact: Actually, CDC reports that acute hepatitis B has been declining in incidence since 1990 mainly due to effective vaccination strategies, with the number of reported cases remaining stable since 2009. Chronic HBV infection, on the other hand, remains a major public health challenge. More than one-half of chronic HBV infections are among Asians and Pacific Islanders, and 71.3 percent are among persons born outside of the U.S. In addition, approximately 47 percent to 70 percent of those with HBV infection living in the U.S. were born in other countries.12
Myth: Hepatitis B can be inherited.
Fact: Yes and no. While hepatitis B can’t be inherited from parents, pregnant women infected with HBV can spread the virus to their babies during childbirth.12
Myth: Hepatitis B can be contracted by eating contaminated food or by kissing.
Fact: The only way hepatitis B can be spread is by coming into contact with blood, semen and vaginal secretions. The most common causes of transmission include unprotected sexual contact, sharing needles among injection drug users and reuse of contaminated needles and syringes. Other causes of transmission include sharing of razor blades or toothbrushes. In the U.S. and Canada, the virus is rarely transmitted via blood transfusions since donated blood is routinely screened for hepatitis B.6
Myth: People with hepatitis B have very obvious symptoms.
Fact: People can live with hepatitis B for decades without having any symptoms or feeling sick. It is estimated that only 30 percent of people who are infected with the virus show any signs or symptoms.12 And, two out of three Asian-Americans with hepatitis B don’t know they are infected.13 Those who do show symptoms usually do so within 90 days after exposure. Symptoms may include jaundice, dark-colored urine, tan-colored stools, mild fever, excessive tiredness, poor appetite, abdominal pain, nausea and vomiting.10,14
Myth: Hepatitis B affects all persons the same.
Fact: The natural course of hepatitis B differs from one person to another. When individuals are initially infected, they have acute hepatitis B infection. During the acute phase, 90 percent of adults’ immune systems will successfully fight it, clearing the infection within six months, healing the liver completely and becoming immune to hepatitis B infection for the rest of their lives. Not as fortunate, 10 percent of adults’ immune systems are unable to fight the virus, and they develop chronic hepatitis B infection, meaning they will have it for the rest of their lives. In babies, the percentages are reversed; only 10 percent clear the infection, while the remaining 90 percent develop chronic hepatitis B infection.
In those with chronic hepatitis B infection, the liver becomes inflamed and scarred over the years, but the speed at which that takes place varies between people. Some will not have a problem during their lifetime, while others will develop severe liver scarring (cirrhosis) within 20 years.15 Left untreated, nearly one in four people with chronic hepatitis B develops serious liver problems, even liver cancer.13 Indeed, hepatitis B is the main cause of liver cancer.14 And, it is a leading cause of cancer deaths among Asian-Americans.13
Myth: People with hepatitis B will likely develop other strains of hepatitis.
Fact: It’s not possible for hepatitis B to develop into another strain of hepatitis because each is caused by its individual strain. For instance, hepatitis A is caused by the hepatitis A virus, and so on. However, sometimes people with hepatitis B also get hepatitis D.12
In observance of World Hepatitis Day 2016, the Hepatitis B Foundation partnered with Eiger BioPharmaceuticals, a developer of therapeutics for hepatitis D, to launch a first-of-its-kind hepatitis D disease awareness and testing program. Hepatitis D is the deadliest form of viral hepatitis that occurs only in people already infected with hepatitis B since the D virus needs the B virus to survive. “Hepatitis delta is the most severe form of viral hepatitis, yet many patients suffering from chronic hepatitis B are unaware of their risk for co-infection with hepatitis delta virus,” said Robert Gish, MD, medical director of the Hepatitis B Foundation. It is hoped that expanded testing will enable patients and their healthcare providers to better understand disease progression and clinical management outcomes.16
Myth: There is no reliable protection against HBV infection.
Fact: Controlled clinical trials show that the hepatitis B vaccine provides greater than 90 percent protection from acute and chronic infection in infants, children and adults immunized before being exposed to the virus.17 And, immunologic memory provided by the vaccine remains intact for at least 20 years among healthy individuals who initiate the vaccine on or after 6 months of age. Studies also show that the vaccine provides long-term protection against clinical illness and chronic HBV infection even though antibody levels might become low or decline below detectable levels.11
Currently, there are two single-antigen and two combination vaccines available in the U.S. Single-antigen vaccines, which protect against only hepatitis B virus, include Engerix-B (GlaxoSmithKline) and Recombivax (Merck), both of which can be administered to infants and adults. Combination vaccines include Pediarix (GlaxoSmithKline), which protects against hepatitis B, diphtheria, tetanus, pertussis and inactivated poliovirus, and Twinrix (GlaxoSmithKline), which protects against both hepatitis A and B. Pediarix is for children only, ages 6 weeks through 7 years. Twinrix is recommended only for persons aged 18 years or older who are at increased risk for both hepatitis A and B infections.11
Both CDC and the American Academy of Pediatrics recommend that all children receive the hepatitis B vaccine starting at birth. CDC also recommends the vaccine for people who are at increased risk of contracting HBV (Table 2).8
The recommended vaccine schedule for both children and adults is three intramuscular injections, with the second and third doses administered one month and six months, respectively, after the first dose. Infants born to HBV-infected mothers require the vaccine and hepatitis B immune globulin within 12 hours of birth to protect them from infection. And, because the vaccine contains no live virus, it is safe during pregnancy and lactation, as well as in immunocompromised persons.11

Myth: Hepatitis B can be treated.
Fact: Generally, there is no treatment for acute hepatitis B because it usually resolves in a matter of weeks. In this case, individuals are advised to get plenty of rest, adequate nutrition and fluids. In severe cases, some people need to be hospitalized.18
Treatment for chronic hepatitis B includes antiviral drugs and regular monitoring for signs of liver disease progression. Currently, there are seven FDA-approved antiviral drugs to treat chronic HBV infection (Table 3): 19
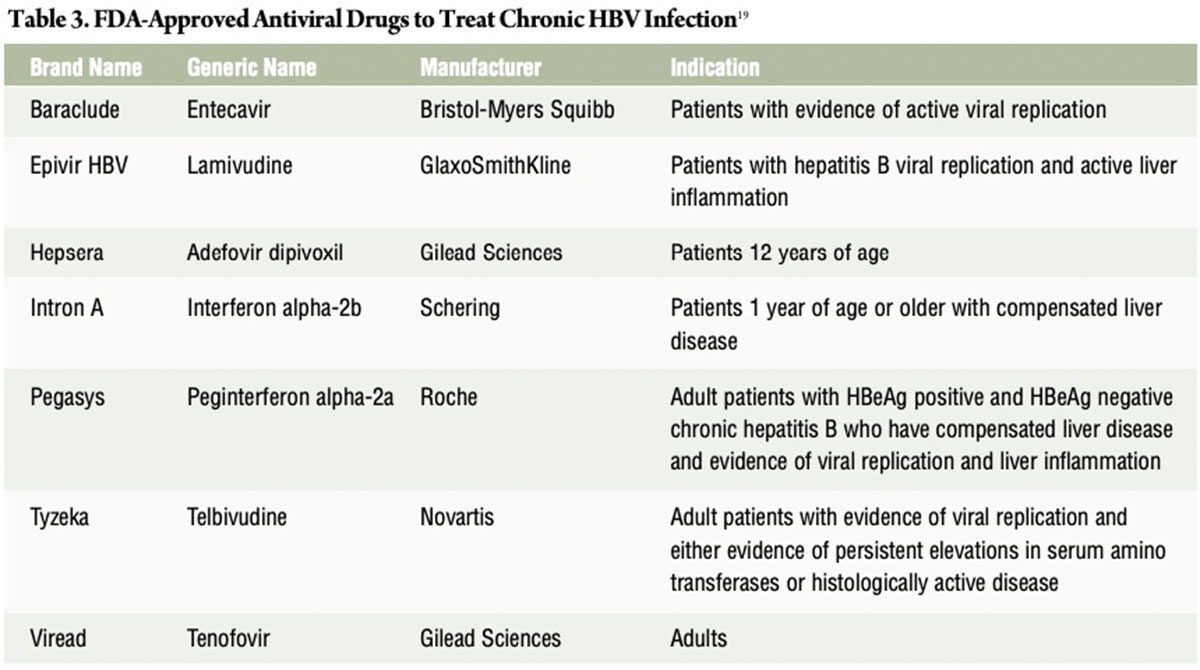
In 2015, the World Health Organization issued its first hepatitis B treatment guidelines that cover the full spectrum of care, from determining who needs treatment, to what medicines to use and how to monitor people long-term (Table 4).20

Myth: Hepatitis B can be cured.
Fact: Acute hepatitis B usually cures itself when the body kills the virus. There is, however, no cure for chronic hepatitis B, only treatment, although medicines may lower virus levels in the body to an undetectable level.21 There may be hope for a cure in the future, though.
In April 2015, Australian scientists reported on a study in which they used an experimental U.S.-created cancer drug to cure hundreds of mice suffering from chronic hepatitis B. The drug, birinapant, has been tested in more than 350 Americans, but it is still in clinical testing. “Birinapant enabled the destruction of hepatitis B-infected liver cells while leaving normal cells unharmed,” said Marc Pellegrini, who led the team at Melbourne’s Walter and Eliza Hall Institute. “Excitingly, when birinapant was administered in combination with the current antiviral drug entecavir, the infection was cleared twice as fast compared with birinapant alone.”
According to Pellegrini, the drug restores apoptosis, a biological mechanism that clears damaged cells. “Normally, [healthy] liver cells would respond to infection by switching on a signal that tells the cell to destroy itself for the greater good, preventing further infection,” he explains. “However, our research showed that the virus commandeers the liver cells’ internal communications, telling the cells to ignore the infection and stay alive. Birinapant flips the cell survival ‘switch’ used by the virus, causing the infected cell to die.”
Birinapant, developed by Tetralogic, has been undergoing medical tests since 2009 and is currently in Phase II trials. Human clinical trials of birinapant in combination with entecavir are currently underway in Melbourne, Perth and Adelaide.22
Also working toward a cure, the Hepatitis B Foundation, announced in January its successful completion of its “Nobel Challenge” campaign that raised more than $3 million to further its mission to eliminate the deadly hepatitis B virus. Funds will support the efforts of its nonprofit research arm, the Baruch S. Blumberg Institute, to accelerate the pace of research in pursuit of a cure for hepatitis B. In March, the Institute recruited a team of nationally renowned scientists to focus exclusively on research to develop a cure for hepatitis B.
According to the Foundation, “Blumberg researchers are building on recent discoveries that have heightened the momentum around finding a cure for hepatitis B and liver cancer: new screening methods to search for effective drugs; new ways to treat hepatitis B using different approaches to shut down the virus; a new blood biomarker that aids in the early detection of liver cancer; and a promising drug that selectively kills liver cancer cells in animal studies.” The researchers, according to Timothy Block, PhD, president and co-founder of the Hepatitis B Foundation, “are among the first, if not the only group, to identify a small molecule that inhibits hepatitis B virus cccDNA formation. This is significant because inhibition of cccDNA is considered essential in achieving a complete cure.” The scientists hope their breakthrough discoveries will result in human clinical trials within the next three years.23,24
Dispelling the Myths Now
On July 28, World Hepatitis Day was celebrated with the theme “elimination.” According to the World Health Organization (WHO), 2016 is a pivotal year for viral hepatitis with WHO member states adopting an elimination strategy for the disease.25 The initiative, which was introduced at the 69th World Health Assembly in May, is the first global health sector strategy on viral hepatitis and includes goals for the elimination of viral hepatitis as a public health threat by 2030.26
According to CDC’s Division of Viral Hepatitis, the National Academies of Sciences, Engineering and Medicine Health and Medicine Division (formerly the Institute of Medicine) released a report that explores the barriers to eliminating the public health problem of hepatitis B and hepatitis C in the U.S., and reaffirms that hepatitis elimination can be achieved with the right resources, commitment and strategy. Its follow-up (Phase II) report, due to be released in early 2017, is expected to include specific recommendations and targets for elimination.
Until these efforts come to fruition, with roughly one-third of the global population infected with HBV, heightening awareness and eliminating the myths surrounding hepatitis B are the best measures for limiting this deadly disease.
References
- McArthur G. Over 650 Melbourne Patients Facing Hepatitis B Scare. Herald Sun, April 13, 2016. Accessed at www.heraldsun.com.au/news/victoria/over-650-melbourne-patients-facing-hepatitis-b-scare/news-story/020fc1febcb0eb622506ee34346393c3.
- Thousands in New Jersey at Risk for Hepatitis B and HIV. Lawyers and Settlements, April 2, 2009. Accessed at www.lawyersandsettlements.com/lawsuit/medical-malpractice-new-jersey-hep-b-scare.html?utm_expid=3607522-13. Y4u1ixZNSt6o8v_5N8VGVA.0&utm_referrer=http%3A%2F%2Fwww.bing.com%2Fsearch%3Fq%3Dhepatitis% 2Bb%2Bscare%26first%3D11%26FORM%3DPORE.
- Candiotti S, Levitt R, and Lavandera E. W. Scott Harrington HIV, Hepatitis Scare Update: Tulsa Dentist’s Patients Waiting for Test Results. WPTV, April 1, 2013. Accessed at www.wptv.com/news/national/w-scott-harringtonhiv-hepatitis-scare-update-tulsa-dentists-patients-waiting-for-test-results.
- Hepatitis B Virus. The History of Hepatitis B. Accessed at www.hepatitisbvirus.net/the-history-hepatitis-b.php.
- Coalition to Eradicate Viral Hepatitis in Asia Pacific. A Brief History of Hepatitis. Accessed at cevhap.org/index.php/en/about-viral-hepatitis/a-brief-history-of-hepatitis.
- Hepatitis B Research Network. About Hepatitis B. Accessed at www.hepbnet.org/about.asp.
- The Hepatitis B Virus Page. Introduction & History. Accessed at hepatitisbviruspage.com/history.htm.
- Centers for Disease Control and Prevention. The ABCs of Hepatitis. Accessed at www.cdc.gov/hepatitis/Resources/Professionals/PDFs/ABCTable.pdf.
- Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis — United States, 2014. Accessed at www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm.
- Health Hub. Hepatitis B (Myths). Accessed at www.healthhub.sg/a-z/diseases-and-conditions/48/HepatitisB.
- Centers for Disease Control and Prevention. Hepatitis B FAQs for Health Professionals. Accessed at www.cdc.gov/hepatitis/HBV/HBVfaq.htm.
- Hepatitis B Is One of the Most Common Infectious Diseases in the World: Myths vs. Facts. Accessed at hepatitisinfo.com.au/understanding-hepatitis-b/myths-vs-facts.
- Centers for Disease Control and Prevention. Know Hepatitis B. Accessed at www.cdc.gov/knowhepatitisb/index.htm.
- WebMD. Hepatitis HealthCenter. Accessed at www.webmd.com/hepatitis/hepb-guide/hepatitis-b-topic-overview.
- Communicable Disease Control and Prevention. Hepatitis B. Accessed at www.sfcdcp.org/hepatitisb.html.
- Hepatitis B Foundation Partners with Eiger BioPharmaceuticals to Raise Awareness of Hepatitis Delta Virus Co-Infection. Hepatitis B Foundation press release, July 28, 2016. Accessed at www.hepb.org/assets/Uploads/2016-07-28-HBF-and-Eiger-World-Hepatitis-Day-Release.pdf.
- Margolis HS. Testimony on Hepatitis B Vaccine Before the House Committee on Government Reform, Subcommittee on Criminal Justice, Drug Policy, and Human Resources, May 18, 1999. Accessed at www.hhs.gov/asl/testify/t990518b.html.
- LiverTox. Acute Hepatitis. Accessed at livertox.nih.gov/Phenotypes_acutehepatitis.html.
- U.S. Food and Drug Administration. Hepatitis B and C Treatments. Accessed at www.fda.gov/ForPatients/Illness/HepatitisBC/ucm408658.htm.
- WHO Issues Its First Hepatitis B Treatment Guidelines. WHO press release, March 12, 2015. Accessed at www.who.int/mediacentre/news/releases/2015/hepatitis-b-guideline/en.
- Cure for Hepatitis B.eMedTV. Accessed at hepatitis.emedtv.com/hepatitis-b/cure-for-hepatitis-b.html.
- New Drug ‘100 Percent Successful’ in Treating PreviouslyIncurable Hepatitis B. RT News, April 22, 2015. Accessed at www.rt.com/news/252129-hepatitis-drug-trial-australia.
- Hepatitis B Foundation’s ‘Nobel Challenge’ Raises $3 Million to Support Research for a Cure. Hepatitis B Foundation press release, January 2016. Accessed at www.hepb.org/assets/Uploads/Nobel-Challenge-CompletedJan.-2016-3-.pdf.
- Hepatitis B Foundation. Hepatitis B: Is a Cure Possible? Accessed at www.hepb.org/news-and-events/freenewsletters/hepatitis-b-is-a-cure-possible?
- Debnath B. Creating Awareness Is the Only Way of Eliminating Hepatitis. MedIndia, July 27, 2016. Accessed at www.medindia.net/news/creating-awareness-is-the-only-way-of-eliminating-hepatitis-162111-1.htm.
- Centers for Disease Control and Prevention. The CDC/Division of Viral Hepatitis: Bringing Together Science and Public-Health Practices for the Elimination of Viral Hepatitis. Accessed at www.cdc.gov/hepatitis/featuredtopics/dvhstrategicplan.htm.