Transforming Hemophilia Care: A New Generation of Extended Half-Life Factor Concentrates
- By Keith Berman, MPH, MBA
For the first time, long-lasting clotting factor therapy provides the possibility of a simpler, more durable treatment regimen for persons with hemophilia.
— Margaret V. Ragni, MD, MPH
FOR CHILDREN AND adults with hemophilia and a history of spontaneous bleeding episodes, the old “on-demand” strategy of reactively administering clotting factors to treat bleeds was displaced more than 20 years ago by a new standard of care: routine prophylactic infusions to maintain coagulation function and prevent bleeds from occurring in the first place. Prophylaxis is well-proven as a means to avoid development of crippling hemarthroses and normalizing the daily lives of persons with hemophilia, who can travel without fear and participate in previously off-limits physical activities.
But there is a catch. The very short circulating half-life of natural or recombinant coagulation factors necessitates frequent infusions. For children with hemophilia A, who metabolize infused factor VIII (FVIII) more rapidly than adults, dosing is typically necessary every other day to assure that the FVIII trough level remains above the critical lower threshold (1 to 3 percent or higher) required for protective hemostatic function. As factor IX (FIX) has a somewhat longer circulating half-life, it is most common for persons with hemophilia B to dose themselves twice per week.* This necessity to frequently infuse factor on a lifelong basis has created its own set of management challenges:
Problems with peripheral venous access in children. Many younger children have peripheral veins that are very small, hard to find or tend to roll away, making them very difficult to “hit” with the small gauge needle on the first try. Repeated multiple times each week, this translates into much stress for both the child and the parent caregiver. To address this problem, the physician will usually offer the option of surgically placing a central venous access device (CVAD), such as a port, to facilitate simple and reliable infusions.
CVAD-associated infection and thrombosis risks. Even with meticulous adherence to sterile technique, use of a CVAD to administer factor creates significant risks of a local or, more seriously, systemic sepsis. Each infusion additionally introduces the potential for thrombosis that clogs the port or other CVAD. The more frequently factor must be administered each week, the more likely it is that parents will give up on administering the product through peripheral veins — with negligible risk of infection or thrombosis — and opt instead for placement and use of a CVAD that entails both risks.
Treatment burden. Regardless of age, the need to prepare and administer factor multiple times each week on a chronic basis is unpleasant, and for some patients and caregivers can lead to treatment “exhaustion.”
Poor adherence to a demanding prophylaxis regimen. This can pose a particular problem for adolescent and young adult males, at a life stage where it is commonplace to question authority and to underestimate the health risks of their behaviors and life choices. The more demanding and burdensome the prophylaxis regimen, the more likely it is not adhered to, with associated higher risks of hemarthroses and long-term joint damage.
The technical feasibility and obvious advantages of FVIII and FIX treatments able to persist longer in the circulation spurred a host of new product development programs, both by leading manufacturers of hemophilia therapies and by biopharmaceutical industry newcomers to hemophilia. For the most part, they have applied technologies already used successfully to produce other types of long-acting therapeutic proteins.
Multiple Means to Extend Factor Half-Life
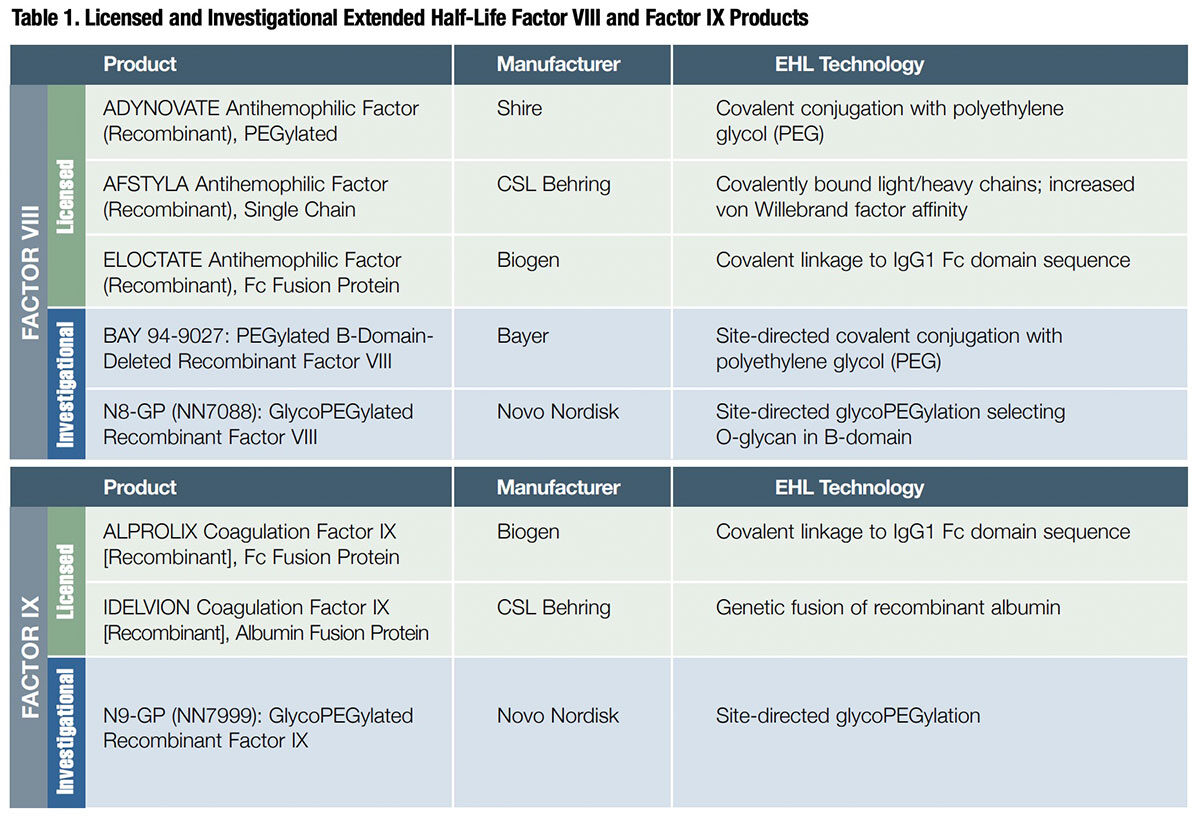
Three fundamental approaches have been exploited to produce the five extended half-life (EHL) recombinant FVIII and FIX products now on the market, and several others currently in development (Table 1):
- Attachment of polyethylene glycol (PEG) to the molecule (PEGylation or glycoPEGylation);
- A natural vascular endothelial cell-mediated process that “recycles” immunoglobulin G (IgG) and albumin into the circulation; and
- Specifically for FVIII, molecular engineering to stabilize the protein and increase von Willebrand factor affinity.
Both PEGylation (applied by Shire and Bayer) and glycoPEGylation (Novo Nordisk) prolong intravascular half-life of FVIII and FIX by virtue of the ability of long protein-bound strands of PEG to 1) prevent uptake and clearance by reticuloendothelial cells, 2) decrease the formation of neutralizing antibodies by masking antigenic sites and 3) protect against proteolysis by enzymes such as trypsin, chymotrypsin and proteases.1
Products developed by Biogen and CSL Behring exploit a physiologic process that enables IgG antibodies and albumin — which together account for 80 percent to 90 percent of total plasma protein — to persist in the bloodstream for an average of about three weeks. Both proteins are bound by a neonatal Fc receptor (FcRn) on the surface of endothelial cells lining the blood vessels, then endocytosed and subsequently recycled into the bloodstream. The two companies employed chemistries to attach either the Fc receptor of IgG1 or recombinant human albumin to FVIII or FIX, which then piggyback on the natural endocytosis-mediated IgG or albumin recycling process.
And, in an entirely novel approach, CSL Behring covalently linked the light and heavy chains of FVIII to form a more intrinsically stable single chain. This single-chain FVIII variant has a roughly two-fold higher affinity for von Willebrand factor, whose primary role is to prevent the premature activation and clearance of the protein.
EHL Factor IX Persists Longer in Circulation
As different as these bioengineered clotting factors are, the half-life extension they achieve is surprisingly similar: roughly 1.5- to 1.6-fold for EHL FVIII products and three- to five-fold for EHL FIX products.
Accounting for roughly 80 percent of all persons with hemophilia, hemophilia A is around four times more prevalent than hemophilia B.2 Yet thanks to the much longer half-life of EHL FIX — ranging from 80 to 110 hours in adults versus under 20 hours for EHL FVIII — the benefits of EHL technologies are more pronounced for the smaller hemophilia B population with a bleeding tendency severe enough to warrant prophylaxis.
While recommended starting dosages and infusion intervals differ somewhat for each of the three approved EHL FVIII products, prophylaxis is commonly started and maintained with twice-weekly dosing. In two pivotal Phase III clinical studies evaluating Shire’s ADYNOVATE and Biogen’s ELOCTATE, the median dosing interval for subjects assigned to an adaptable prophylaxis dosing regimen was about 3-1/2 days.3,4
By contrast, hemophilia B patients initiating EHL FIX prophylaxis typically start with once-weekly dosing. In 26 clinical study subjects on Biogen’s ALPROLIX product,5 whose treatment interval was adjusted to maintain FIX trough level between 1 percent and 3 percent above baseline or higher as clinically indicated to prevent bleeding, the median interval between infusions was just shy of 14 days. Similarly, the majority of 37 subjects who completed six months of prophylaxis therapy with CSL Behring’s IDELVION FIX at a dose ≤40 IU/kg switched successfully to a 14-day interval at dose ranging from 50-75 IU/kg.6
The comparatively modest FVIII half-life extension attained with modifications similar to EHL FIX (e.g., PEGylation and FcRn-mediated endothelial recycling) can be attributed to the absence of von Willebrand factor (VWF) in the bioengineered FVIII protein; unfortunately, natural VWF that complexes with infused EHL FVIII has a relatively short half-life itself.7
Absent some entirely new bioengineering breakthrough, it is unlikely that EHL FVIII products will ever approach the seven- to 14-day dosing interval now routinely enjoyed by hemophilia B patients on EHL FIX prophylaxis. Nevertheless, most children and nearly all adults on standard FVIII prophylaxis are able to reduce their number of infusions by at least one per week after switching to an EHL FVIII product.8
Adjusting the Regimen to Fit the Need
For many patients, it is quite satisfactory to adhere to the recommended dosing frequency — twice-weekly infusions of EHL FVIII, for example — with dose adjustments to achieve a trough level of 1 percent to 3 percent above baseline or as clinically indicated to prevent bleeding. But for some patients, it appears advantageous to individualize the regimen, adjusting the dose or the dosing interval based on their prior prophylaxis experience with standard factor concentrates, the trough level goal and actual breakthrough bleeding experience.
The vast majority of patients starting EHL factor prophylaxis were already on a prophylaxis regimen with standard recombinant factor concentrates. The presumptive justification to switch for most of these individuals is the reduced treatment burden of a prolonged interval between infusions. But converting to an EHL product also presents an additional opportunity for individualizing dosing frequency in a way that places a priority on protection from spontaneous bleeds.
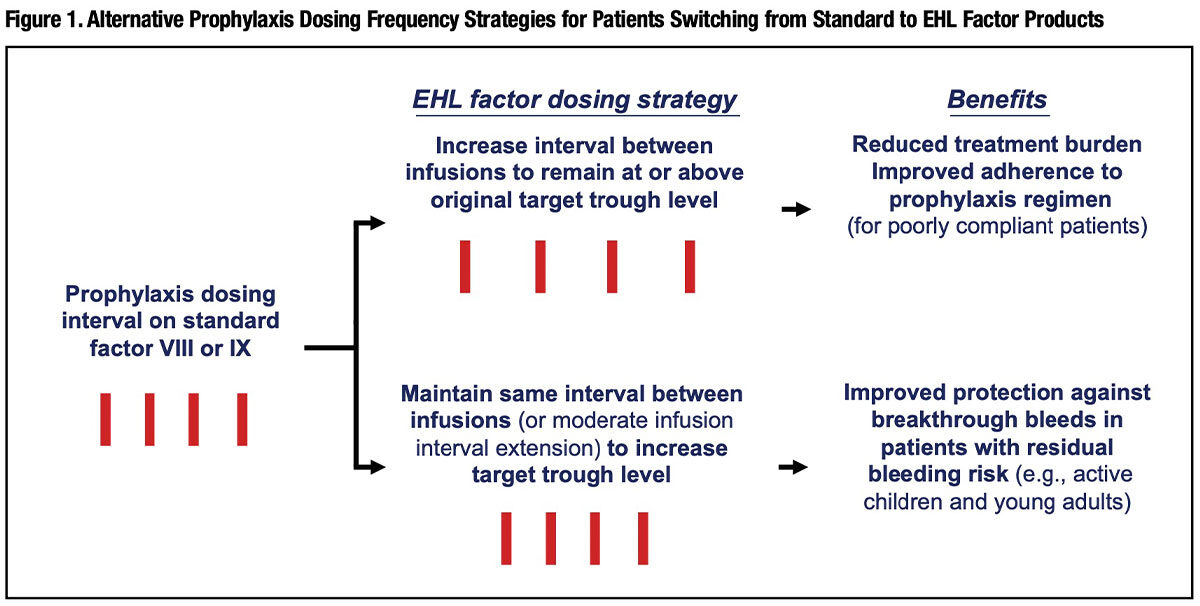
Figure 1 outlines two optional strategies for prophylaxis-managed patients who make the switch to EHL products. For the majority of patients with a history of very good bleeding protection on standard factor prophylaxis, an EHL product allows the physician to extend the interval between infusions, reducing the chronic treatment burden and potentially improving adherence for those with a poor treatment compliance history. But for patients with less-than-satisfactory control of spontaneous bleeds on their original prophylaxis regimen — physically active children and adolescents, for example — the physician may wish to opt for maintaining the same infusion interval (or reducing its extension) with the goal of attaining a higher, more protective trough level.9
The dose itself can also be adjusted to increase the trough level, but there are limits. This is true in particular for EHL FVIII products with their relatively modest half-life advantage over standard FVIII. There is a temptation for patients who still require twice or three times weekly infusions to simply boost the dose as a means to extend the dosing interval. Unfortunately, this strategy has a serious downside. The pharmacokinetics of this clotting factor dictates that, beyond a point, overall product utilization — and cost — escalates unacceptably when the interval between doses is stretched and the dose is increased to try to sustain a protective trough level. “Increasing the dose size rather than reducing dose frequency is an inherently inefficient approach to therapy,” Professor Michael Laffan at London’s Hammersmith Hospital pointed out in a recent review.10
Embracing the Payoffs of EHL Products
A survey of 25 hemophilia treatment centers (HTCs) conducted earlier this year by The Marketing Research Bureau (MRB)11 revealed that 13 percent of hemophilia A patients on prophylaxis therapy now use an EHL FVIII product. Unsurprisingly, given their much longer seven- to 14-day dosing interval, nearly 37 percent of hemophilia B patients on a prophylaxis regimen are now administering an EHL FIX product.
Demand for EHL products is expected to continue to grow as clinicians, patients and caregivers recognize their ability to:
- Reduce treatment burden on children and caregivers using peripheral veins for prophylaxis therapy;
- Offer a more acceptable alternative to use of CVADs in younger children, with their attendant risks of infection and line clogging;
- Improve adolescent/young adult compliance with their prescribed prophylaxis regimen, potentially translating into fewer hemarthroses and reduced risk of joint damage;
- Reduce patient resistance to switching from episodic treatment to prophylaxis, reducing bleeding event rates and long-term joint damage risk; and
- Facilitate targeting of higher, more protective trough levels, with attendant reduction in breakthrough bleeding risk.
There are already indications that EHL products are enjoying broadening appeal. In contrast to results from an earlier survey of HTCs conducted in late 2014, MRB’s latest 2016 survey found that the reduced burden of administering EHL factors is now attracting significant numbers of patients who had been episodically treating spontaneous bleeding events.
In the near future, we can expect to see publication of patient studies quantifying the benefits of extended half-life factor concentrates and providing guidance on how to best integrate them into clinical practice. But in the meantime, all indications are that this new product class represents a powerful new tool for improving the care and quality of life for persons with hemophilia.
* The frequency of infusion is highly individual, depending on variables that include age (half-life of infused factors is shorter in children than adults), severity of the factor deficiency, dosage and experience with breakthrough bleeds.
References
- Pisal DS, Kosloski MP, Balu-lyer SV. Delivery of therapeutic proteins. J Pharm Sci 2010 Jun;99(6):2557-75.
- Soucie JM, Evatt B, Jackson D. Occurrence of hemophilia in the United States. Am J Hematol 1998 Dec;59(4):288-94.
- ADYNOVATE [Antihemophilic Factor (Recombinant), PEGylated]. Full prescribing information; November 2015.
- ELOCTATE [Antihemophilic Factor (Recombinant), Fc Fusion Protein]. Full prescribing information; January 2016.
- ALPROLIX [Coagulation Factor IX (Recombinant), Fc Fusion Protein]. Full prescribing information; February 2016.
- IDELVION [Coagulation Factor IX (Recombinant), Albumin Fusion Protein]. Full prescribing information; March 2016.
- Young G, Mahlangu JN. Extended half-life clotting factor concentrates: results from published clinical trials. Haemophilia 2016 Jul;22 Suppl 5:25-30.
- Frampton JE. Efmoroctocog alfa: A review in haemophilia A. Drugs 2016 Aug 3 [Epub ahead of print].
- Pipe SW. The hope and reality of long-acting hemophilia products. Am J Hematol 2012 May;87 Suppl 1:S33-9.
- Laffan M. Extended half-life productsin factorVIII: benefitsand limitations. Haemophilia 2014 Jul;20 Suppl 5:1-20.
- TheMarketingResearchBureau,Inc.(Orange,CT). Survey onHemophilia Care&Price Monitoring—United States(Wave#23). June 2016.