The Escalating Diabetes Epidemic
Will the evolving therapeutic guidelines for treating diabetes, as well as managing its risks and complications, stem the tide of this costly and sometimes deadly disease?
- By Tina Tockarshewsky
Diabetes continues to be a complex chronic illness epidemic defined by staggering statistics expressed in millions, if not billions. According to the Centers for Disease Control and Prevention’s (CDC) 2014 National Diabetes Statistics Report, 29.1 million people (or 9.3 percent of the U.S. population) have diabetes; of these, 8.1 million are undiagnosed (27.8 percent).
Year after year, the numbers escalate exponentially. Currently, it is estimated that:
- 1.7 million new cases of diabetes are diagnosed each year (in adults 20 years or older);
- 86 million adults (greater than one in three) are pre-diabetic; and
- 15 percent to 30 percent of those with pre-diabetes will develop type 2 diabetes within five years.
The prevalence of diabetes (as well as pre-diabetes) is also increasing across age groups and races/ethnicities. Type 1 diabetes, once thought of as a childhood disease, has begun to appear in adults; type 2 diabetes, once a chronic illness of adulthood, has been trending younger into the childhood years at alarming rates. While diabetes can be found in all demographics, its presence is growing across and within races and ethnicities:
- 15.9 percent of American Indians/Alaska Natives adults
- 13.2 percent of non-Hispanic black adults
- 12.8 percent of Hispanic adults
- 9 percent of Asian-American adults
And the numbers continue to escalate.
The Cost of Diabetes
Using 2012 data, CDC estimates this diabetes epidemic costs the U.S. $245 billion in combined direct and indirect costs. Direct medical costs in 2012 were $176 billion, a number 2.3 times higher than costs for non-diabetics; indirect costs come in at an alarming $69 billion due to disability, work loss and premature death. Many of these numbers are feared to be an underestimation of the actual human and economic toll.
Case in point, only 35 percent to 40 percent of people with diabetes actually had diabetes noted on their death certificates, and approximately 10 percent to 15 percent had diabetes listed as cause of death — yet, the risk of death for those with diabetes is 50 percent greater than it is for those without diabetes. Thankfully, for those deaths reported, CDC can track diabetes-related death rates falling up to 40 percent between 1997 and 2006 as a result of progressive improvements in cardiovascular care, glucose management and lifestyle interventions.1
Picking Up the Pace of the Therapeutic Pipeline
The therapeutic guidelines for treating diabetes, as well as managing its risks and complications, are continually being refined as a result of research and outcomes. Diagnostic benchmarks and tools also are evolving and improving. While there have been great strides made in recognizing that disease management involves healthy lifestyle changes (such as diet and exercise regimens) in conjunction with medications, it is also well-documented that medications — and improved adherence to medication regimens — increase health outcomes, as well as reduce risks and their associated costs. According to a study published in the American Journal of Managed Care,2 medication adherence by diabetes patients results in fewer diabetes-related complications, causing fewer amputations/ulcers (4 percent lower), fewer renal events (5 percent lower), less neuropathy/nerve damage (4 percent lower) and less retinopathy/eye damage (2.7 percent lower). Additionally, a study published in Health Affairs indicates that better medication adherence by patients could prevent more than one million emergency department visits and hospitalizations each year — an annual healthcare cost savings of $8.3 billion.3
A quick “diabetes” word search on www.clinicaltrial.gov generates a listing of nearly 3,000 active clinical trials across the type 1 and type 2 chronic care and symptom management spectrum. The Pharmaceutical Research and Manufacturers of America’s (PHRMA) 2014 Report on Medicines in Development: Diabetes cites 180 new medicines in development (Phase I, II, III and applications submitted) for type 1 and type 2 diabetes and diabetes-related conditions.4 This pipeline also includes 110 medications that may benefit older adults, of particular note since diabetes impacts 10.9 million Americans over age 65.2
New Therapies = New Approaches
Eight new classes of type 2 diabetes medications have been added by the U.S. Food and Drug Administration (FDA) in the last few years. The current pipeline being tested will hopefully expand the therapeutic toolkit even further by developing new medications to potentially offer:
- Improvements in glucose-dependent insulin secretion
- Diabetic nerve pain relief, including a medication to block an enzyme associated with diabetic neuropathy (nerve damage)
- Stimulation and enhancement of insulin-producing cell regeneration
- Next-generation oral treatments
- Once-weekly treatments
- Glucose regulation for type 2 by a delayed-release formulation of metformin that acts as a gut sensory modulator (GSM)
- First-in-class therapy to protect against and treat diabetic nephropathy (chronic progressive kidney disease)4
While the market awaits these and other trial outcomes, in February, FDA gave expanded use approval for Lucentis (ranibizumab injection) 0.3 mg as the first drug to treat diabetic retinopathy (DR) in patients with diabetic macular edema (DME). A once-a-month eye injection administered in a physician’s office, Lucentis is intended for use in conjunction with other appropriate control therapies. With diabetes being the leading cause of new blindness for people ages 20 to 74 years, diabetic retinopathy is a significant diabetes complication and the most common diabetic eye disease (33 percent of diabetics older than 40 have diabetic retinopathy). Lucentis had been previously approved to treat DME, which led to two clinical studies being conducted to test the drug’s safety and efficacy in treating DR with DME. Strong early evidence caused FDA to fast track the drug’s approval for DR, finally offering people with diabetes access to their first retinopathy therapy.5
Heightened emphasis on improving quality of life for those diagnosed has yielded significant new therapies within the scope of the past two years. FDA has already approved several unique therapies to expand the clinician’s toolkit for improving diabetes care (see Figure 1).
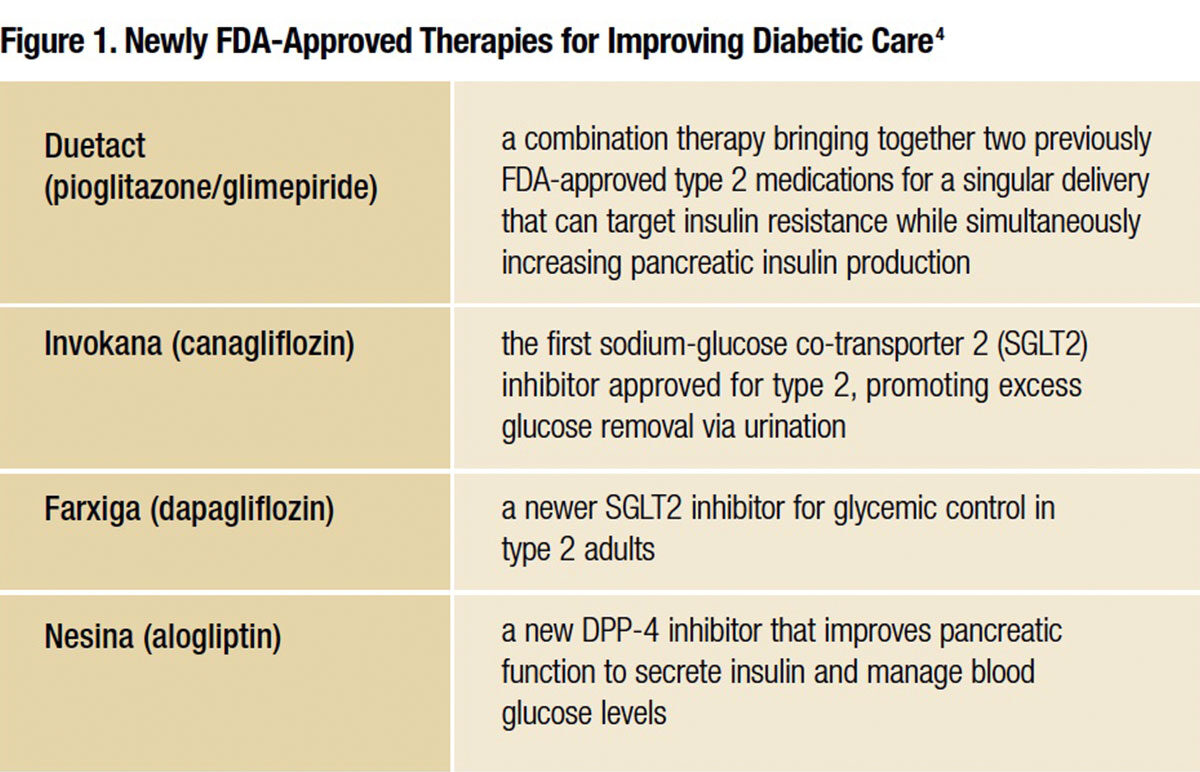
Resolving DPP-4 Concerns
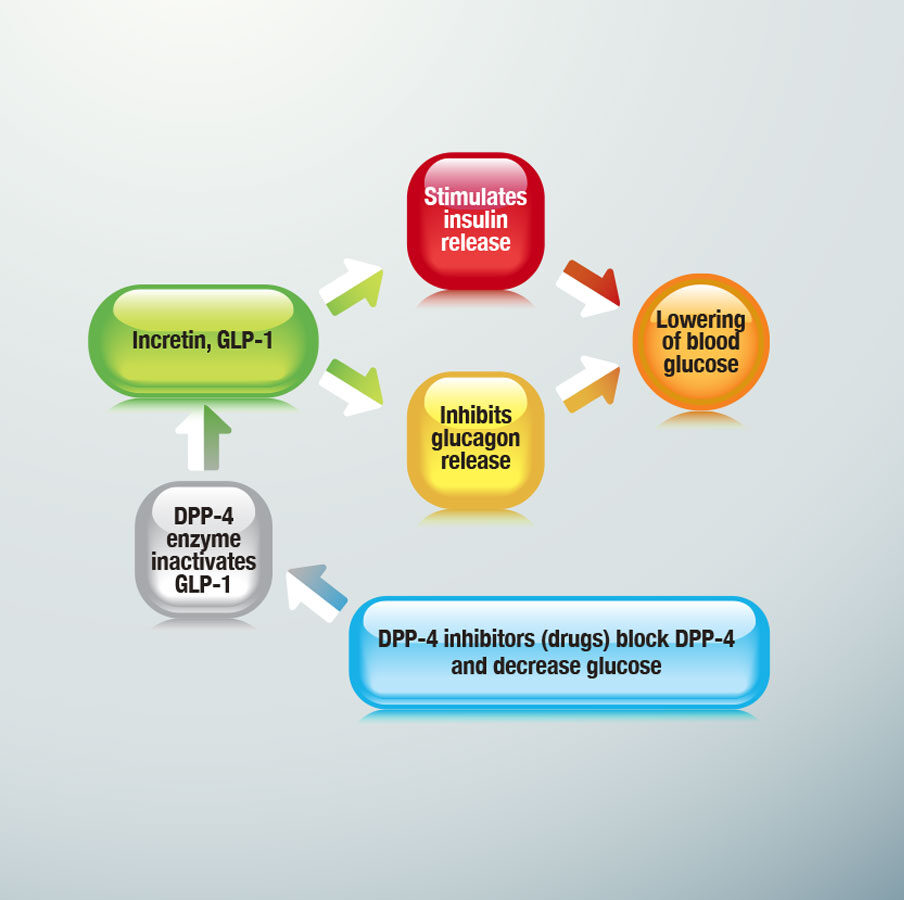
Inhibitors of dipeptidyl peptidase 4, known as DPP-4 inhibitors, are a class of oral hypoglycemics used to treat type 2 diabetes (see Figure 2). Despite their promise as newer frontline therapies, all of the DPP-4 inhibitors, like alogliptin (Nesina) and sitagliptin (Januvia), are currently receiving close examination due to cardiovascular events documented across this class of medication. Those in the diabetes field are anxiously awaiting a soon-to-be completed cardiovascular-outcomes trial for Januvia use, with results due to be presented in June at an American Diabetes Association (ADA) meeting. Outcomes of the trial, called TECOS, are expected to be particularly telling because they specifically test Januvia, the DPP-4 inhibitor that has been available and used for the longest period of time. This trial follows on the heels of two other large DPP-4 inhibitor outcomes trial studies, Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)-TIMI 53 and EXAMINE with alogliptin, both of which yielded unexpected cardiovascular events indicative of a possible increase in risk for heart failure in type 2 patients taking these medications. SAVOR-TIMI 53 results had a hazard ratio of 1.27; P = .007 for hospitalization for heart failure in patients taking saxagliptin vs. placebo, resulting in FDA announcing it would more closely review saxagliptin’s heart failure rate. EXAMINE also yielded a pattern of higher heart failure hospitalization risk for diabetic patients on alogliptin. Other sitagliptin studies before TECOS have produced conflicting heart failure results. Experts still believe DPP-4 inhibitors are generally safe for diabetes patients with heart failure unless the patient has a history of advanced heart failure and concomitant renal failure. With mixed results and serious concerns documented, the June TECOS report will be significant for patients, professionals and the pharmaceutical industry alike as all try to assess the future for DPP-4 inhibitors.6
Do Exchanges Restrict Access to Therapies?
Access to care is a crucial part of successful diabetes treatment and management. With more therapeutic options available (and hopefully more on the horizon), medication adherence is a strong driver of health outcomes. Yet, socio-economic factors hindering diagnoses, as well as policies and provider coverage issues, may contribute to roadblocks for accessing therapies. A PHRMA analysis of 84 health insurance exchange plans in the 15 states with the highest expected 2014 exchange enrollment found some plans created significant barriers to access for diabetes therapies. These included the exclusion of certain types of medicines from the formulary, high co-insurance rates for some diabetes medications, and a greater likelihood for diabetes medications to face step therapy or prior authorization challenges in the exchanges (compared with employer or benchmark plans).
With co-insurance of more than 40 percent required for antidiabetics 21 percent of the time and more than 40 percent for insulins 16 percent of the time, resulting annual out-of-pocket costs for patients could be from $195 to $1,150 for antidiabetics and $600 to $4,000 for insulins. Variations in plans from state to state not only risk inconsistencies in care, but the analysis showed that the states anticipated to have the highest exchange enrollments do not cover many diabetes medications: Nine of these top 15 states cover less than 60 percent of single-source diabetes medicines on the market, with plans in Georgia, Indiana and Ohio, N.Y., in particular covering, on average, less than half of the single-sourcemedicines available.7
Modifications to Care Management and New Standards of Care
The base of knowledge for improvements in clinical care for diabetes continues to expand. Because cardiovascular disease (CVD) is the primary cause of death associated with diabetes and the leading contributor to diabetes costs, its risks of heart attacks and stroke become a critical part of the overall management and prevention treatment plan for people with diabetes. Of all the common diabetes comorbidities leading to CVD risk, hypertension management has been shown to be one of the strongest areas for health improvements.
In the Feb. 10 issue of JAMA, researchers show that blood pressure-lowering treatment for type 2 patients results in a lower CVD risk, fewer heart disease events and improvements in mortality rates. People with diabetes, on average, have higher blood pressure (BP) levels; however, there currently exists a debate over which patients warrant BP-lowering therapy and which BP targets to use for benchmarking. In the JAMA article, researchers note that every 10 mmHg lower systolic BP demonstrated a lower risk of mortality, CVD events, heart attacks, stroke, albuminuria (excess protein in the urine) and diabetic retinopathy. Although there were proportional health gains realized for most outcomes when the systolic BP level was brought to 140 mmHg, the data reported that bringing the level down further to below 130 mmHg provided even lower risk for stroke, retinopathy and albuminuria. This possibly indicates that people at high risk for these complications could greatly benefit from the BP reduction.8
Because of the chronic care complexities of managing diabetes, in 2012, ADA published a consensus document reviewing all relevant literature to provide clinical practice recommendations. That document has been subsequently updated in the recent January “Standards of Medical Care in Diabetes — 2015.” While broken down into 14 areas of focus, the document is meant to be viewed in its entirety for evidence-based and expert opinions on best practices and guidelines for care.
In acknowledging the complexities, the document offers the following strategies for improving care:
- “A patient-centered communication style that incorporates patient preferences, assesses literacy and numeracy, and addresses cultural barriers to care should be used;
- Treatment decisions should be timely and founded on evidence-based guidelines that are tailored to individual patient preferences, prognoses, and comorbidities;
- Care should be aligned with components of the Chronic Care Model (CCM) to ensure productive interactions between a prepared proactive practice team and an informed activated patient; and,
- When feasible, care systems should support team-based care, community involvement, patient registries, and decision support tools to meet patient needs.”9
The standards stress that inherent to these strategies are three themes clinicians, policymakers and advocates need to be mindful of: patient centeredness, diabetes across the life span and advocacy for people with diabetes.
Practicing patient centeredness recognizes that recommendations are, indeed, just recommendations: The clinician needs to consider the particular needs and risks for each individual patient when developing individualized plans of care. Diabetes across the life span acknowledges the challenge of managing and coordinating care between clinical teams as patients transition through different stages of their lives (including all stages of pregnancy). It also recognizes that diabetes is trending younger, and concedes that many older adults are living longer with chronic diabetes — a demographic for which there is a lack of clinical trial evidence to help guide therapy use. Advocacy promotes the need to help patients access lifestyle improvements that can prevent diabetes or help with quality of life for those diagnosed. Especially since lifestyle factors like weight management, physical activity and smoking cessation can have huge health impacts — and socioeconomic factors can become barriers to diagnosis, care and access to these programs — advocacy becomes critical to connect patients with programs that can directly impact their health.
In updating the 2015 standards, several key revisions are worth noting because they reflect new research and changes in expert opinion:
- Classification and diagnosis of diabetes: For obese Asian-Americans, the body mass index cutoff point for screening for pre-diabetes and type 2 diabetes changed to 23 kg/m2 (vs. 25 kg/m2), reflecting current evidence that this population (vs. the general population) is at a greater risk for diabetes at lower BMI levels.
- Foundations of care: Education, nutrition, physical activity, smoking cessation, psychosocial care and immunization:
-Given new evidence that all people, including those with diabetes, should limit the amount of time they are sedentary to be less than 90 minutes spent sitting, the physical activity section encourages patients to break up extended amounts of sedentary time.
-With the increasing popularity of e-cigarettes, the standards point out that e-cigarettes are not considered a smoking alternative or cessation tool.
-Immunization guidelines now reflect CDC guidelines for PCV13 and PPSV23 vaccinations in older adults.
- Glycemic targets: ADA recommendations for pre-meal blood glucose targets are now 80 mg/dL to 130 mg/dL, rather than 70 mg/dL to 130 mg/dL as a result of new data comparing actual average glucose levels with A1C targets.
- Cardiovascular disease and risk management: To reflect evidence from randomized clinical trials, the recommended goal for diastolic blood pressure management was changed from 80 mmHg to 90 mmHg for most people with diabetes and hypertension. Statins treatment and lipid monitoring recommendations were adjusted to treatment initiation (and initial statin dose) being driven by risk status rather than LDL cholesterol level. Lipid screening profile is recommended at diabetes diagnosis, at initial medical evaluation and/or at age 40, and periodically after that.
- Microvascular complications and foot care: Foot examinations during every clinical visit are encouraged, especially for those with insensate feet, foot deformities or history of foot ulcers, to identify those at high risk for foot-related complications.
- Children and adolescents:With new evidence indicating the importance of tight glycemic control in children and adolescents with diabetes, a target of A1C of less than 7.5 percent is recommended for all pediatric age groups, with individualization still being encouraged.
- Management of diabetes in pregnancy: A newly added section addressing pregnancy provides recommendations from pre-conception through delivery regarding care and diabetes management.9
Initial Evaluation and Diabetes Management Planning
The standards also extensively address the many diabetes complications and comorbidities. Some of the recommendations include screening, as appropriate, those with type 1 diabetes for autoimmune diseases (e.g., thyroid dysfunction, celiac disease), plus encouragement to assess for common co-morbid conditions (e.g., depression, obstructive sleep apnea, fatty liver disease, fractures, cancer, cognitive impairment, low testosterone in men, periodontal disease and hearing impairment) that may complicate diabetes management.
Not included in the standards is a new study released Jan. 8 in Diabetes Care citing the possibilities of using a procedure called corneal confocal microscopy (CCM) to predict diabetic peripheral neuropathy (DPN). Researchers used CCM to assess deficits in corneal nerve fiber length (CNFL) in 90 non-neuropathic type 1 patients over the course of four years and then assessed who did and did not develop DPN. They found that the receiver operator characteristic curve could be used to determine measures of neuropathy to predict DPN. While CCM has been previously used in assessing DPN, the researchers were pleased to discover the ability of CCM to predict DPN, which expanded the diagnostic capabilities of this novel ophthalmic marker.10 “Confocal microscopy (CCM) holds great potential as a diagnostic tool for peripheral neuropathy in clinical trials,” concurs A. Gordon Smith, MD, director of the University of Utah’s Peripheral Neuropathy Clinic and Cutaneous Innervation Laboratory and vice chair of research for the department of neurology. “With diabetic peripheral neuropathy, the biggest challenge for research and developing treatments is that the focus continues to be primarily symptomatic for pain rather than focusing on disease alteration. For this reason, we’re looking at the expansion of lifestyle interventions to address painful diabetic peripheral neuropathy.”
The University of Utah team has recently published findings in both the Annals of Neurology (January 2015) and the Annals of Clinical and Translational Neurology (October 2014) linking exercise directly to possible disease modifications for DPN.11,12 Exercise was found to increase cutaneous nerve density in diabetic patients without neuropathy, plus exercise resulted in the clear ability for cutaneous axons to regenerate following controlled denervation, with the exercise actually enhancing nerve regeneration rates.
Early diabetic neuropathy involves the loss of unmyelinated axons; this causes pain, numbness and progressive deterioration of intraepidermal nerve fiber density (IENFD). The Utah team found that when patients with type 2 diabetes — but without neuropathy — were given therapeutic interventions of either lifestyle counseling or weekly exercise for one year, the exercise cohort demonstrated significant increase in distal leg IENFD, yet the counseling cohort remained the same. Not only does this indicate that damage to unmyelinated axons could be prevented in pre-diabetic conditions, it also demonstrated that IENFD may become a useful biomarker for future clinical trials assessing prevention.
In yet another approach to tracking IENFD involvement, a study of the unmyelinated cutaneous axons was conducted using the premise that these axons are not only susceptible to physical and metabolic injury, but they also are very capable of rapid regeneration. Metabolic syndrome served as the test since it demonstrates reduced baseline IENFD and cutaneous regeneration comparable to rates seen in diabetes. A short but intense six-month exercise program designed to improve glucose, insulin and lipid metabolism resulted in a clear increase in the ability of cutaneous axons to regenerate following controlled denervation. Reduced A1C levels were the primary identifiable individual metabolic result of the exercise intervention, and most strongly correlated with the enhanced regenerative capacity of the axons. Because the study showed significant regeneration after only a short period of exercise intervention, the promise of exercise to prevent and treat diabetic nerve damage and associated pain is highly encouraging.
A Hopeful Future for Managing Diabetes
Just as the diabetes numbers continuously evolve, so, too, does the rate of research and development to stem the tide of the diabetes epidemic. The significant amount of work being done across the field and the improvements in care and treatment are also harbingers of a more hopeful future in managing this complex chronic illness. Still, bringing this epidemic under control and seeking prevention for millions from diabetes’ path of destruction and death is critical. Progress is being made, but time will tell whether that progress can outpace the diabetes juggernaut.
References
- Centers for Disease Control and Prevention. 2014 National Diabetes Statistics Report. Accessed at www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html.
- Pharmaceutical Research and Manufacturers of America. Medicines in Development for Older Americans-The Medicare Population and Leading Chronic Diseases: 2014 Report. Accessed at www.phrma.org/sites/default/files/pdf/2014-meds-in-dev-older-americans.pdf.
- Ashish JK, Aubert RE, Yao J, Teagarden JR, and Epstein RS. Greater Adherence to Diabetes Drugs Is Linked to Less Hospital Use and Could Save Nearly $5 billion Annually. Health Affairs, August 2012, 31:81836-1846. Accessed at content.healthaffairs.org/content/31/8/1836.abstract#cited-by.
- Pharmaceutical Research and Manufacturers of America. 2014 Report: Medicines in Development: Diabetes. Accessed at www.phrma.org/sites/default/files/pdf/diabetes2014.pdf.
- U.S. Food and Drug Administration. FDA Approves Lucentis to Treat Diabetic Retinopathy in Patients with Diabetic Macular Edema. Press release, Feb. 6, 2014. Accessed at www.fda.gov/NewsEvents/NewsroomPressAnnouncements/ucm433392.htm.
- Nainggolan L. TECOS Study with Sitagliptin to Be Reported at ADA Meeting. Medscape Medical News, Feb. 5, 2014. Accessed at www.medscape.com/viewarticle/839315.
- Pharmaceutical Research and Manufacturers of America. Access to Diabetes Medicines in Exchange Plans Report. Accessed at www.phrma.org/sites/default/files/pdf/exchanges-diabetes.pdf.
- Williams B. Treating Hypertension in Patients With Diabetes: When to Start and How Low to Go? JAMA. 2015;313(6):573-574. Accessed at jama.jamanetwork.com/article.aspx?articleid=2108870.
- American Diabetes Association. Strategies for Improving Care. Sec. 1 in Standards of Medical Care in Diabetes—2015. Diabetes Care, 2015;38(Suppl. 1):S4-87. Accessed at professional.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/January%20Supplement%20Combined_Final.pdf.
- Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, and Efron N. Corneal Confocal Microscopy Predicts 4-Year Incident Peripheral Neuropathy in Type 1 Diabetes. Diabetes Care published ahead of print Jan. 8, 2015. Accessed at care.diabetesjournals.org/content/early/2015/01/01/dc14-2114.abstract.
- Singleton JR, Marcus RL, Lessard MK, Jackson JE, and Smith AG. Supervised Exercise Improves Cutaneous Reinnervation Capacity in Metabolic Syndrome Patients. Annals of Neurology, 77: 146–153. Accessed at onlinelibrary.wiley.com/doi/10.1002/ana.24310/pdf.
- Singleton JR, Marcus RL, Jackson JE, Lessard MK, Graham TE, and Smith AG. Exercise Increases Cutaneous Nerve Density in Diabetic Patients Without Neuropathy. Annals of Clinical and Translational Neurology, 1: 844–849. Accessed at onlinelibrary.wiley.com/doi/10.1002/acn3.125/pdf.