Subcutaneous Immune Globulin: New Therapeutic Uses Beyond Primary Immunodeficiency?
While the intravenous route has been the standard method of IG therapy for autoimmune and other neuromuscular disorders, recent studies show that the subcutaneous route is both as effective and more preferred by patients.
- By Keith Berman, MPH, MBA
While they have been available and increasingly popular in Europe over the last 25 years as replacement therapy for persons with primary immunodeficiency disorders (PIs),1 the first subcutaneous immune globulin (SCIG) product wasn’t approved for marketing in the U.S. until 2006. As recently as 2011, there was but a single SCIG brand — rather inconveniently formulated at the same 16 percent concentration as intramuscular immune globulin. Early on, some physicians recommended SCIG for carefully selected PI patients, but the vast majority continued to receive intravenous immune globulin (IVIG) every three to four weeks in a hospital outpatient clinic or physician office setting.
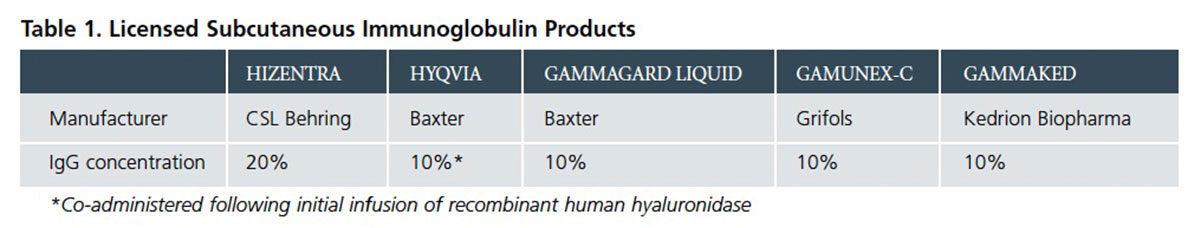
Thanks in part to a wave of published comparative studies, reviews and commentaries over the last several years, more U.S. immunologists and infectious disease specialists have become aware of specific advantages of SCIG in relation to IVIG for qualifying and motivated PI patients. Today, physicians can select from among five approved SCIG product options for their patients (Table 1), with at least two others reported to be in development. A steadily growing proportion of PI patients who require lifelong IG replacement therapy are newly initiating or switching to SCIG therapy, drawn in part by the obvious appeal of self-administering their therapy at home in lieu of hospital, office clinic or home nursing visits to receive IVIG therapy.
With the benefit of years of experience with these products, investigators across Europe more recently have reported that SCIG may represent a better treatment option for patients chronically managed with IVIG for certain autoimmune neuromuscular diseases and secondary immunodeficiency disorders at risk for serious bacterial infections. Could self-administered SCIG represent a better option than IVIG for some patients with these disorders as well?
What Makes SCIG a Better Option for Some PI Patients?
To answer this question, it is important to consider why SCIG self-administration — typically using a programmable syringe infusion pump2 — continues to attract a growing following among PI treatment specialists and patients.
Non-serious systemic adverse reactions are much less frequent with SCIG than IVIG therapy. Typically, smaller more frequent doses of SCIG act to moderate spikes and troughs in IgG serum concentration. The high supra-physiologic serum IgG peak that occurs immediately following IVIG infusion likely contributes to a two- to three-fold higher incidence of non-serious systemic adverse reactions — mainly headache, fatigue, pyrexia, chills, nausea and vomiting — than is seen following SCIG infusion.3,4,5 Roughly one-half of patients newly starting SCIG experience generally minor local infusion site reactions — far more often than occurs with IVIG administration — but typical redness and swelling is transient and usually declines over time.
Risk of serious systemic adverse reactions is exceedingly low for currently licensed SCIG preparations. While serious systemic adverse events associated with IVIG therapy — primarily aseptic meningitis, thrombosis and hemolysis — are very uncommon (and often preventable with premedication and slowing of the infusion rate), they do occur and have been reported both in published clinical studies and retrospective chart reviews. By contrast, no serious systemic adverse reactions have occurred to date in licensing trials conducted for any currently available SCIG product. These findings are consistent with a number of published case series. Recent U.S. and Swedish studies in 47 and 60 PI patients, respectively, reported a combined total of more than 4,000 home-based SCIG infusions with no serious systemic adverse events.6,7 An earlier large Scandinavian study documented just six moderate adverse systemic reactions and no severe or anaphylactoid reactions in 165 patients who received a total of 33,168 SCIG infusions.8 Simply put, opting for SCIG therapy reduces a very low risk of serious systemic adverse events to an extremely remote risk.
SCIG offers an equally effective treatment option for patients with IVIG tolerability or venous access problems. A very small but significant proportion of patients repeatedly experience very unpleasant or debilitating adverse reactions to IVIG, which cannot be managed with premedication, a reduction in infusion rate or a change of product brand. Patients occasionally present with veins that are very difficult to access, necessitating surgical implantation of indwelling catheters that introduces its own potential infection risks. For these patients who are willing and capable of self-infusing at home, SCIG represents a safe and simple option to resolve these issues and continue to receive the full protective benefit of IG therapy.
SCIG self-administration permits dosing schedule flexibility and results in fewer lost work and school days. IVIG therapy generally necessitates a clinic visit every three to four weeks. For non-elderly patients, those scheduled visits — including the infusion itself and post-infusion recovery and observation time — translate into lost work and school days. Self-administration of SCIG, by contrast, can be flexibly scheduled after school or work, during evenings or on weekends. Multiple studies have quantified important reductions in lost school and work days after switching from IVIG to SCIG.9
Patients who experience SCIG therapy consistently prefer it over IVIG. IVIG may be a better option for PI patients with good venous access who tolerate the product well, and who variously have poor manual dexterity, lack of motivation to take responsibility for self-treatment, or have expressed reluctance to deal with needles or the mechanics of self-infusion. But surveys of other patients who were able to switch to SCIG consistently document a strong preference for SCIG therapy (Table 2), as well as improved health-related quality of life (HRQL) measures.

An additional consideration when choosing between these two options is, of course, cost. Consistent with several European reports, two recent Canadian studies identified significant annual cost savings associated with home self-infusion of SCIG, driven by near-elimination of the need for infusion nursing and ancillary personnel.10,11 These analyses are predicated on dosing SCIG equivalently to IVIG, a practice that also prevails in Europe. In the U.S., initial dosing instructions for four of the five available SCIG products* recommend boosting the total SCIG dose either by 37 percent (Gammagard Liquid, Gamunex-C and Gammaked) or by 53 percent (Hizentra) — with the objective of equalizing the estimated “area under the curve” (AUC) that corresponds with total circulating IgG over a specified period of time. However, available evidence, including a recent crossover study, suggests that dose-equivalent therapy with SCIG is as effective as IVIG for protection of PI patients against serious infections.12 Possibly, the pharmacokinetics of SCIG — characterized by much less fluctuation in serum IgG and a significantly higher mean serum IgG trough level than IVIG — may help to offset the lower AUC profile of a dose-equivalent SCIG regimen.
SCIG for Autoimmune Neuromuscular Disorders and More
The multiple demonstrated advantages of SCIG therapy for qualified PI patients have not been lost on specialists who often prescribe IVIG as long-term maintenance therapy to manage certain autoimmune inflammatory neuromuscular disorders. Again, the Europeans have been out in front in investigating the feasibility of SCIG for a number of important conditions for which IVIG is already an established first-line therapy.
Chronic inflammatory demyelinating polyneuropathy (CIDP).
Interest in SCIG as a treatment option for this disorder was signaled in 2008 with publication of case reports describing its effectiveness and good tolerability in CIDP patients already successfully managed with IVIG.13,14 In 2013, Danish investigators reported on a study that randomized 30 adult patients successfully managed on maintenance therapy with IVIG to either SCIG at a total dose corresponding to their pre-study IVIG dose or to subcutaneous saline.15 The SCIG group actually experienced a modest increase in isokinetic muscle strength of 5.5 ± 9.5 percent (P < 0.05) as compared with an expected decline of 14.4 ± 20.3 percent (P < 0.05) in the saline placebo group. Various other key functional measures similarly improved following SCIG in relation to saline placebo.
This same Danish group then followed 17 CIDP patients, all of whom had previously responded to IVIG, for one year on SCIG maintenance therapy. SCIG preserved muscle strength and functional abilities.16 “SCIG should be considered as an alternative in long-term treatment of CIDP patients,” they concluded.
Very recently, an Italian research team has reported sustained clinical efficacy, as measured by the Overall Neuropathy Limitation Scale (ONLS), with SCIG therapy in a group of 66 CIDP patients previously managed with IVIG (P= 0.018). Just one subject experienced a worsening of symptoms over the four-month study period. Patients additionally reported an improvement in relation to IVIG therapy in their perception of the therapeutic setting.17
With support from CSL Behring, an ambitious 350-subject multicenter, prospective, randomized, double-blind, placebo-controlled trial now in progress will not only try to affirm the therapeutic equivalence of its 20 percent SCIG product, Hizentra, to IVIG, but will attempt to answer whether more aggressive maintenance dosing provides additional clinical benefit. Participating study sites in 15 countries are randomizing subjects with IVIG-dependent CIDP to receive “low dose” or “high dose” weekly SCIG infusions of 0.2 g/kg or 0.4 g/kg body weight, or to placebo infusions. This study is expected to be completed in November 2015.
Multifocal motor neuropathy (MMN). Hypothesizing that an equivalent dosage of SCIG is as effective as IVIG in patients with IVIG-responsive MMN, Danish investigators completed a randomized crossover study in nine subjects.18 The two treatments were equally effective, with SCIG use additionally sparing subjects from “end-of-dose weakness” episodes that some experienced with IVIG therapy. Patient preference findings, however, were not in line with those expressed by PI patients, who strongly favor SCIG. Four of the nine subjects in this small MMN study preferred SCIG, three had no preference and two preferred IVIG. The reason could be traced at least in part to the study protocol: subjects had to self-infuse two to three times weekly.
A UK study of seven MMN patients on stable IVIG dosing who completed six months of once-weekly SCIG treatment again documented no change in muscle strength, disability, motor function or health status. With respect to HRQL, all seven rated SCIG home treatment as “extremely good.” The investigators concluded that “MMN patients with stable clinical course on regular IVIG can be switched to SCIG at the same monthly dose without deterioration and with a sustained overall improvement in HRQL.”19 Sustained clinical efficacy with a stable ONLS score was very recently reported in a series of 21 MMN patients, just one experiencing worsening symptoms.17
Other neuromuscular disorders. At present, IVIG is first-line therapy for patients with steroid-resistant dermatomyositis (DM) and polymyositis (PM). Italian investigators recently reported that no relapse of disease occurred during weekly SCIG treatment of seven patients with severe idiopathic DM or PM previously on maintenance IVIG therapy over a median follow-up period of 14 ± 4 months.20 Three of the seven patients were able to discontinue immunosuppressive drug therapy, and all were able to reduce their daily maintenance prednisone dose. A U.S. proof-of-concept study is set to enroll 10 IVIG-naïve adult subjects with DM this year to evaluate both changes in strength from baseline and participant preference for SCIG in relation to IVIG.21
Another newly organized single-center Phase 2 study at the University of Kansas will assess the safety and efficacy of SCIG in 25 subjects with myasthenia gravis who require maintenance IVIG therapy.
Wherever there is an autoimmune neuromuscular disorder or a disease commonly associated with secondary antibody immunodeficiency requiring chronic IVIG therapy, expect to see new case reports and small patient studies going forward. A prime example is the very recently published Italian single-center experience comparing IVIG and SCIG use in 61 patients with hypogammaglobulinemia secondary to chronic lymphocytic leukemia (CLL) and non-Hodgkins lymphoma (NHL). Unsurprisingly, their results closely mirrored findings in PI patient studies: fewer systemic adverse events, significantly higher IgG trough levels, similar effectiveness in reducing infectious events and need for antibiotic coverage, and a decided improvement in quality of life-related parameters after the switch to SCIG.22
Older Age, Higher Dosage May Mean More SCIG Benefit
While SCIG therapy was originally tested in PI patients and has since become well accepted as a similarly effective alternative to IVIG with fewer adverse systemic effects, arguably the benefits of SCIG are even more important for patients with autoimmune neuromuscular disorders who require IG therapy. The PI IG-treated population includes all ages, but skews heavily toward children and younger adults. Patients with inflammatory neuromuscular conditions that require IVIG therapy, most prominently CIDP and MMN, are largely at the other end of the age spectrum. Most CIDP patients are over age 50 years, with many in their 60s, 70s and even 80s.23 The mean age of onset of MMN is 40 years.24 Of course, CLL and NHL patients on IVIG replacement therapy also comprise an older age demographic.
We know that older age and, in all likelihood, higher dose are among important risk factors for rare but well-documented thrombosis events following IG infusions. Persons whose neuromuscular disorders are managed with IVIG are generally older, and their average monthly dosage is at least two-fold higher than the average for persons with PI. In crossover studies, weekly SCIG self-infusions have been consistently preferred by patients and have a sharply lower serious adverse event risk profile than IVIG therapy. The convenience of SCIG for home infusion has taken an important leap forward with the recent approvals of every-two-week dosing for Hizentra and every three-to-four-week dosing with HYQVIA.
As with PI, adoption of SCIG therapy will take time, but there now seems little doubt that it is an advantageous IG delivery option for properly selected patients with neuromuscular and secondary immunodeficiency disorders now chronically managed with IVIG.
References
- Chapel H and Gardulf A. Subcutaneous immunoglobulin replacement therapy: the European experience. Curr Opin Allergy Clin Immunol 2013 Dec;13(6):623-9.
- McFalls K. Choosing an Infusion Pump. IG Living 2011 Apr-May: 42-43. Accessed at www.igliving.com/Assets/IGL/Articles/IGL_2011-04_AR_Choosing-an-Infusion-Pump.pdf.
- Gammagard Liquid [Immune Globulin Infusion (Human), 10%]. Full prescribing information, September 2013. Accessed 11/17/2014 at www.baxter.com/products/biopharmaceuticals/downloads/gamliquid_PI.pdf.
- Gamunex-C [Immune Globulin Injection (Human), 10%]. Full prescribing information, July 2014. Accessed 11/17/2014 at www.grifols-pi.info/inserts/gamunex.pdf.
- HYQVIA [Immune Globulin Infusion (Human), 10% with Recombinant Human Hyaluronidase]. Full prescribing information, September 2014. Accessed 11/17/2014 at www.baxter.com/downloads/healthcare_professionals/products/HYQVIA_PI.pdf.
- Stein MR, Koterba A, Rodden L, et al. Safety and efficacy of home-based subcutaneous immunoglobulin G in elderly patients with primary immunodeficiency diseases. Postgrad Med 2011 Sep;123(5):186-93.
- Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies — a prospective, multinational study. J Clin Immunol 2006 Mar;26(2):177-85.
- Gardulf A, Andersen V, Björkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet 1995 Feb 11;345 (8946):365-9.
- Fasth A and Nyström J. Safety and efficacy of subcutaneous human immunoglobulin in children with primary immunodeficiency. Acta Paediatrica 2007 Oct;96(10):1474-8.
- Martin A, Lavoie L, Goetghebeur M, et al. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfusion Med 2013 Feb;23(1):55-60.
- Gerth WC, Betschel SD, and Zbrozek AS. Implications to payers of switch from hospital-based intravenous immunoglobulin to home-based subcutaneous immunoglobulin therapy in patients with primary and secondary immunodeficiencies in Canada. Allergy Asthma Clin Immunol 2014 May 7;10(1):23.
- Jolles S, Bernatowska E, de Gracia J, et al. Efficacy and safety of Hizentra in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol 2011;141:90-102.
- Lee DH, Linker RA, Paulus W, et al. Subcutaneous immunoglobulin infusion: a new therapeutic option in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 2008 Mar;37(3):406-9.
- Nobile-Orazio E, Gallia F, Tuccillo F, et al. Chronic inflammatory demyelinating polyradiculoneuropathy and multifocal motor neuropathy: treatment update. Curr Opin Neurol 2010 Oct;23(5):519-23.
- Markvardsen LH, Debost JC, Harbo T, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropaty. Eur J Neurol 2013 May;20(5):836-42.
- Markvardsen LH, Harbo T, Sindrup SH, et al. Subcutaneous immunoglobulin preserves muscle strength in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol 2014 Dec;21(12):1465-70.
- Cocito D, Merola A, Peci E, et al. Subcutaneous immunoglobulin in CIDP and MMN: a short-term nationwide study. J Neurol 2014 Nov;261(11):2159-64.
- Harbo T, Andersen H, Hess A, et al. Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trial. Eur J Neurol 2009;16:631-8.
- Misbah SA, Baumann A, Fazio R, et al. A smooth transition protocol for patients with multifocal motor neuropathy going from intravenous to subcutaneous immunoglobulin therapy: an open-label proof-of-concept study. J Peripher Nerv Syst 2011 Jun;16(2):92-7.
- Danieli MG, Pettinari L, Moretti R, et al. Subcutaneous immunoglobulin in polymyositis and dermatomyositis: a novel application. Autoimmun Rev 2011 Jan;10(3):144-9.
- Subcutaneous Immunoglobulin (Hizentra) in Patients With Dermatomyositis: A Proof of Concept Study. Accessed on ClinicalTrials.gov on 11/19/2014 at clinicaltrials.gov/ct2/show/NCT02271165?term=Jefferson+AND+dermatomyositis+AND+SCIg&rank=1.
- Compagno N, Cinetto F, Semenzato G, et al. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: a single-center experience in 61 patients. Haematologica 2014 Jun;99(6):1101-6.
- Chiò A, Cocito D, Bottacchi E, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J Neurol Neurosurg Psychiatry 2007;78:1349-53.
- Lawson VH, Arnold WD. Multifocal motor neuropathy: a review of pathogenesis, diagnosis, and treatment. Neuropsychiatr Dis Treat 2014 Apr 5;10:567-76.
- Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life and treatment satisfaction in North American patients with Primary Immunodeficiency Diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol 2006;26:65-72.
- Fasth A and Nystrom J. Quality of life and health-care resource utilization among children with primary immunodeficiency receiving home treatment with subcutaneous human immunoglobulin. J Clin Immunol 2008;26:400-5.
- Hoffmann F, Grimbacher B, Thiel J, et al. Home-based subcutaneous immunoglobulin G replacement therapy under real-life conditions in children and adults with antibody deficiency. Eur J Med Res 2010;15(6):238-45.