In the Pipeline: Novel Plasma Proteins for Major Cardiovascular Disorders
- By Keith Berman, MPH, MBA
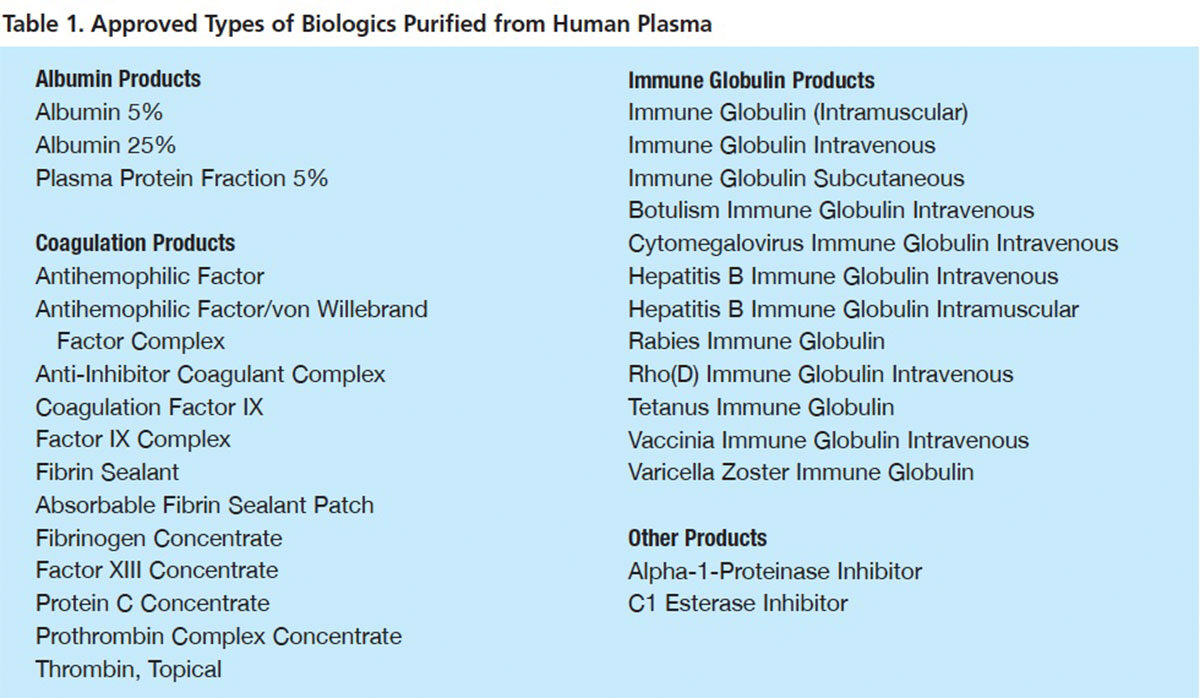
OVER THE 70 years since biochemist Edwin Cohn and his Harvard Medical School laboratory first purified human albumin for use in severe burns and hemorrhagic trauma, nearly 30 therapeutic proteins from human plasma have been purified, proven safe and effective and approved for use. Most of these products (Table 1) are indicated as replacement therapies for rare congenital plasma protein deficiencies or to treat other narrowly defined conditions. But, recently, several leading plasma fractionators have embraced an important new product development focus: identifying and developing new plasma-based protein therapeutics to treat common serious clinical disorders.
Three of the most ambitious of these initiatives hope to exploit the functionality of two proteins purified from human plasma — plasmin and the apolipoprotein A-1 component of high-density lipoprotein (HDL) — both purified from human plasma — to improve health outcomes in patients stricken with acute ischemic stroke, acute peripheral arterial occlusion and acute coronary syndrome.
Human Plasmin: The More Direct Path to Thrombolysis
Composed of cross-linked fibrin and aggregated platelets, a blood clot or thrombus may form following an injury to a blood vessel or as a result of improper activation of hemostasis. When a thrombus blocks more than about 90 percent of the cross-sectional area of an artery, the tissue perfused by that artery will develop anoxia, resulting in infarction. A thrombus that detaches from the artery can cause arterial embolism and potentially lead to infarction in almost any organ in the body.
But it has also long been known that fibrin-containing blood clots can spontaneously degrade on their own. In the late 19th century, the French physiologist Jules Dastre hypothesized that this phenomenon is mediated by a protein enzyme, and coined the term “fibrinolysis” for this process. Finally, in the mid-1940s, the active enzyme that breaks down fibrin (plasmin) and its inert circulating zymogen (plasminogen) were both identified and named by Christensen and MacLeod at New York University.1
The final steps leading to fibrinolysis are straightforward: Plasminogen binds to a blood clot or cell surface and is converted to plasmin through interactions with a number of enzymes. The most important of these is tissue plasminogen activator (tPA). A recombinant version of tPA (Activase, Genentech) is indicated for fibrinolysis — or thrombolysis — in three acute conditions: myocardial infarction, ischemic stroke and pulmonary embolism.
While tPA or urokinase, another plasminogen activator (PA), often are used to treat acute peripheral arterial occlusion (aPAO), they have disadvantages that limit their clinical utility. Because tPA acts one step upstream by converting plasminogen to the active fibrinolytic plasmin, even partial dissolution of the thrombus occluding the peripheral artery can take hours; frequently, tPA doesn’t work at all. The clinical utility of tPA is further limited by the need for physicians to use low doses to minimize the risk of intracranial hemorrhage and other systemic bleeding risks — again slowing and reducing the likelihood of effective clot lysis.
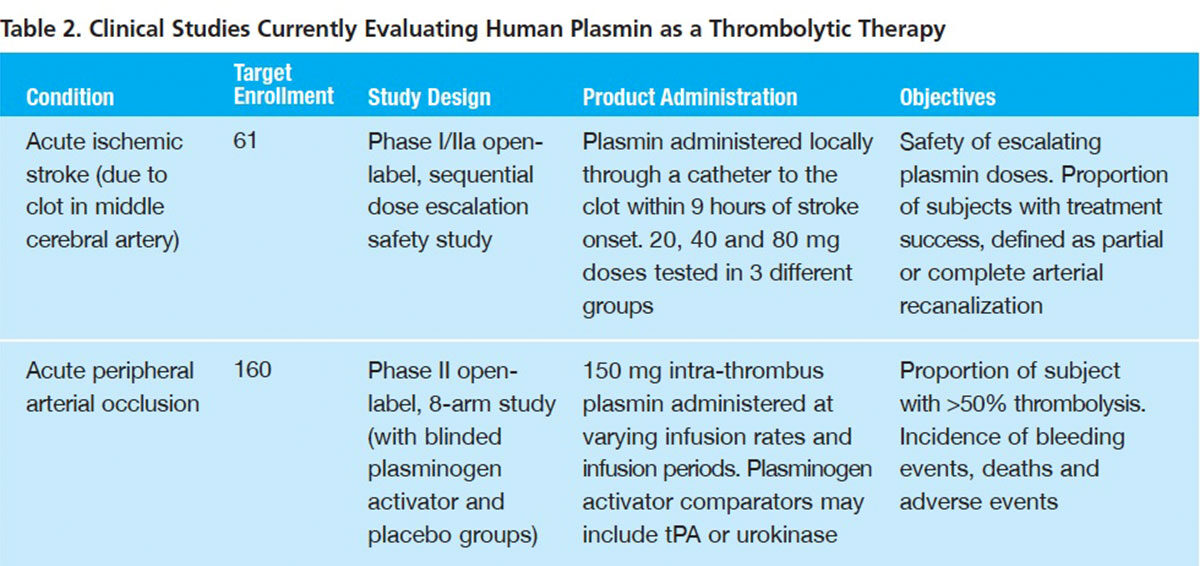
Acute peripheral arterial occlusion (aPAO). The direct action of plasmin to degrade fibrin in arterial thrombi makes it logically appealing for the treatment of aPAO. Preclinical models have documented a striking safety advantage of plasmin over plasminogen activators. In part, this may be attributable to reserves of circulating alpha-2 antiplasmin, which rapidly neutralizes plasmin, allowing higher doses and mitigating the risk of bleeding complications elsewhere in the body.
A recently completed Phase I dose-escalation study sponsored by Grifols evaluated a human plasmin concentrate in 83 patients, in seven dose cohorts ranging from 25 mg to 175 mg, with acute lower extremity arterial or bypass graft occlusion.2 A specially designed balloon catheter was used to more precisely deliver the plasmin to the clot area and thereby minimize the risk of hyperfibrinolysis and bleeding elsewhere in the circulation. Fifty percent or greater thrombolysis occurred in nearly 80 percent of subjects receiving 125 mg to 175 mg of plasmin, as compared with 50 percent who received 25 mg to 100 mg. Major bleeding was infrequent (under 5 percent of subjects) with no trend toward more bleeding at higher dosages of plasmin.
Based on findings from that Phase I trial, investigators are now evaluating a 150 mg plasmin dose in an ambitious Phase II study (Table 2) examining the effect of initial proximal pulse infusion and differing infusion periods and infusion rates on aPAO thrombolysis. In addition to six active treatment arms, patients in this 160-subject trial are also being randomized to receive a plasminogen activator (tPA or urokinase) or saline placebo. Outcomes assessed for up to 30 days post-procedure include:
- Avoidance of open surgical procedures
- Avoidance of amputation
- Avoidance of additional catheter-directed thrombolysis with a PA or mechanical device atherectomy
- Physiological reperfusion (improvement in ankle brachial index)
- Arterial patency (duplex ultrasound imaging)
Roughly 100,000 persons are afflicted annually with aPAO,3 with most cases involving the legs. Despite its slow action and inherent bleeding risk, most patients receive low-dose tPA in an attempt to dissolve the clot, with moderate success. The commercial prospects for plasmin will hinge on whether a pivotal trial can show improved outcomes relative to tPA with a similar or lesser incidence of bleeding complications.
Acute ischemic stroke. Accounting for more than 85 percent of the nearly 800,000 strokes that occur each year in the U.S., ischemic stroke kills more than 130,000 victims and exacts a very large toll in permanent disability. The estimated national cost in healthcare, medications and lost productivity is a staggering $39 billion.4
Brain tissue is particularly sensitive to anoxia: In a typical middle cerebral artery ischemic stroke, an estimated two million nerve cells are lost for each minute that reperfusion is not achieved;5 thus, the well-known principle “time lost is brain lost.” The objective, then, is to at least partly dissolve the arterial clot as rapidly as possible and restore blood flow.
Currently, only tPA is indicated and used for thrombolysis in ischemic stroke. Again, by converting plasminogen into active fibrin-degrading plasmin, tPA can make a critical difference for some patients if administered within three hours — or for selected patients within 4.5 hours6 — after initial stroke symptoms. A pooled analysis of eight trials documented that initiation of tPA thrombolysis within 90 minutes increased the odds of an excellent outcome by 2.6-fold, in the 91- to 180-minute window by 1.6-fold, and in the 181- to 270-minute window by 1.3-fold.7 After that, tPA offers no meaningful benefit, even as the risk of iatrogenic intracerebral or other hemorrhage remains. Only an estimated 100,000 of the nearly 700,000 ischemic stroke victims this year will reach a stroke treatment facility and have imaging studies completed to exclude intracranial hemorrhage within three hours. There is currently no approved drug therapy for treatment of ischemic stroke after three hours.
Assuming it is shown to have acceptably low risk of bleeding complications, there are two ways that plasmin could cut the toll in stroke-related death and long-term disability:
- Effectiveness beyond the three-hour window during which tPA can be utilized following onset of stroke symptoms
- Superior effectiveness in relation to tPA during the three-hour post-onset window period
Grifols and its collaborators in Europe, Australia and the U.S. are currently conducting a Phase I/IIa safety and dose-ranging study in about 60 subjects with acute ischemic stroke localized in the middle cerebral artery. The investigators are evaluating plasmin doses between 20 mg and 60 mg, delivered through a catheter into the thrombus within nine hours of stroke onset.
An estimated 315,000 ischemic stroke patients are diagnosed by qualified stroke specialists between three and nine hours of stroke onset — three times the number who present within the initial three-hour time window.3 An obvious unmet need exists for a safe and effective fibrinolytic for these patients in particular, but whether plasmin is up to the task will, of course, hinge on results of a large, well-designed, controlled clinical trial.
Reconstituted HDL Promising for Acute Coronary Syndrome
High-density lipoprotein (HDL) is actually a combination particle that contains hydrophilic apolipoproteins — mainly apolipoprotein A-1 (apoA-I) — enwrapping hydrophobic cholesterol. A key function of HDL is to facilitate transport of these lipids out of cells in arterial walls to the liver for clearance. Not surprisingly, higher circulating HDL levels are associated with lower risk of atherosclerosis, in particular coronary atherosclerosis that can eventually lead to thrombosis and acute myocardial infarction (AMI).
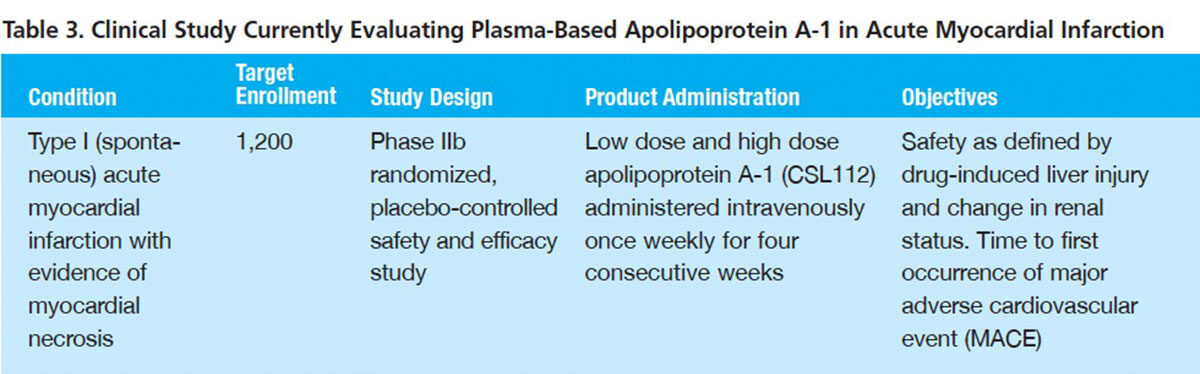
CSL Behring and its collaborators have shown that four once-weekly infusions of an apoA-I purified from donor human plasma dramatically elevate measures indicative of reverse cholesterol transport, while rapidly raising blood levels of apoA-I. This “reconstituted high-density lipoprotein” (rHDL) appears also to powerfully impede inflammation, primarily by inhibiting pro-inflammatory cytokine production.8
Encouraged by these findings, in May, the company initiated a Phase IIb study to evaluate rHDL in patients with AMI and evidence of myocardial necrosis (Table 3). A total of 1,200 subjects will be randomized to receive four infusions of a low dose or high dose of rHDL or placebo. In addition to safety, the time to first occurrence of a major adverse cardiovascular event (MACE) will be captured for the three study arms.
The target population for a pivotal clinical trial may be a subset of patients with acute coronary syndrome (ACS) associated with AMI or unstable angina. Ten percent to 15 percent of patients experience a MACE over the 12 months following an episode of ACS. If short-term administration of rHDL can significantly reduce that rate and it is well tolerated, particularly with respect to liver function, it could become part of the standard treatment armamentarium for many of the estimated 600,000 patients hospitalized with ACS each year.
The Plasma Industry Steps Up Its Game
Evaluating a human plasma protein or any potential new product for complex conditions like ischemic stroke, aPAO and ACS presents special challenges. Outcomes in these major vascular disorders may be influenced by a number of patient demographic variables and underlying comorbidities. There are established treatment regimens against which the candidate plasma protein must be directly compared. To demonstrate safety and efficacy, clinical testing requirements are necessarily more extensive, and subject enrollments significantly larger than, say, clotting factor replacement therapy for a specific hereditary coagulation disorder.
In pursuing these and other bold new product development initiatives, leading manufacturers in the plasma products industry have signaled their willingness to address these challenges, accept the risks and make the necessary investments. Clearly, the potential rewards offer ample justification: the prospect that treatment with purified plasma proteins now discarded as waste can cut the risk of serious morbidity in patients with major debilitating and life-threatening disorders.
References
- Christensen LR and Macleod CM. A proteolytic enzyme of serum: characterization, activation, and reaction with inhibitors. J Gen Physiol 1945 Jul 20;28(6):559-83.
- Marder VJ, Comerota AJ, Shlansky-Goldberg RD, et al. Safety of catheter-delivered plasmin in patients with acute lower extremity arterial or bypass graft occlusion: phase I results. J Thromb Haemost 2012 Jun;10(6):985-91.
- The Marketing Research Bureau, Inc. Novel Plasma Proteins: Scientific and Commercial Potential (September 2013).
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123(8):933-44.
- von Kummer R, Allen KL, Holle R, Bozzao L, Bastianello S, Manelfe C, et al. Acute stroke: usefulness of early CT findings before thrombolytic therapy. Radiology Nov 1997;205(2):327-33.
- Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke Aug 2009;40(8):2945-8.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke. Stroke May 2007;38(5):1655-711.
- Spirig R, Schaub A, Kropf A, et al. Reconstituted high-density lipoprotein modulates activation of human leukocytes. PLOSone 2013 Aug 14;8(8):e71235.