Autoimmune Disease: A More Effective Treatment on the Horizon?
- By Elissa Ritt, MAS
INTRAVENOUS IMMUNE GLOBULIN (IVIG) is a life-changing and, in many cases, life-saving treatment. IVIG is used to treat a multitude of disease states from the more familiar primary immunodeficiencies and autoimmune neuropathies to the more esoteric pemphigus vulgaris and even recurrent miscarriages. Treatment dosages for autoimmune diseases that range from 500 milligrams to 2 grams per kilogram of patient body weight (usually given monthly) result in a massive amount of IVIG being used by the healthcare system. Not only is IVIG a very expensive treatment, but because it is made from a limited resource, shortages have occurred.1 Therefore, researchers are driven to find ways to make IG therapy more effective at smaller doses.
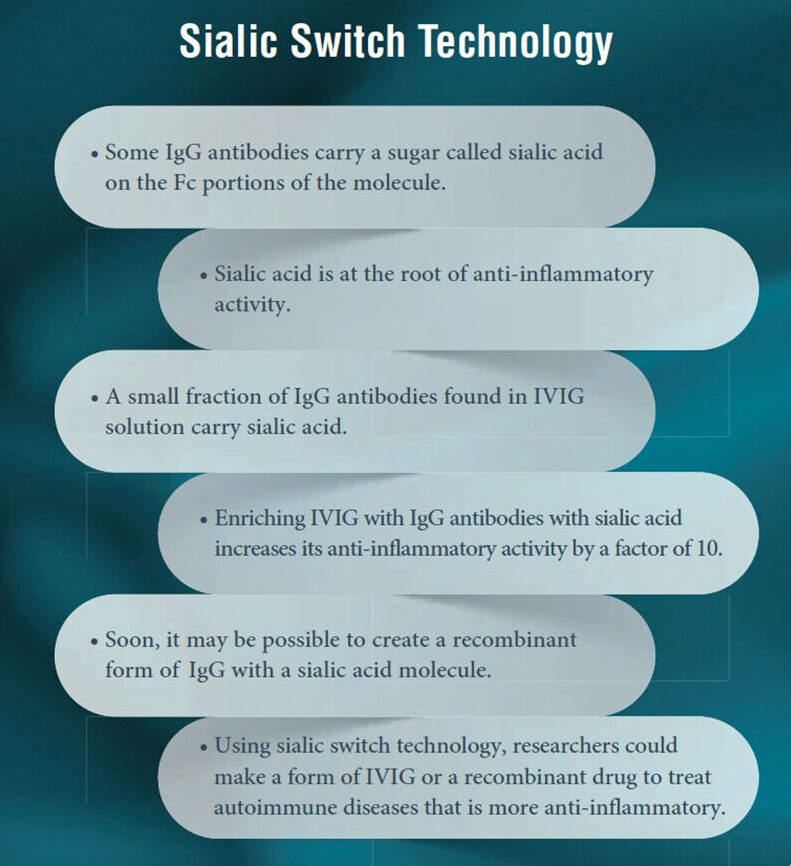
The Sialic Acid Discovery
In 2008, Dr. Jeffrey Ravetch and his team at The Rockefeller University made a molecular discovery that could potentially be used to improve the anti-inflammatory effects of IVIG. Dr. Ravetch noted that a small number of the antibodies found in IVIG are different from the others; they exhibit a greater affinity to receptor sites that, when activated, blunt the immune response. This small, distinct subset of antibodies has a molecular entity called a sialic acid group attached to one end.2
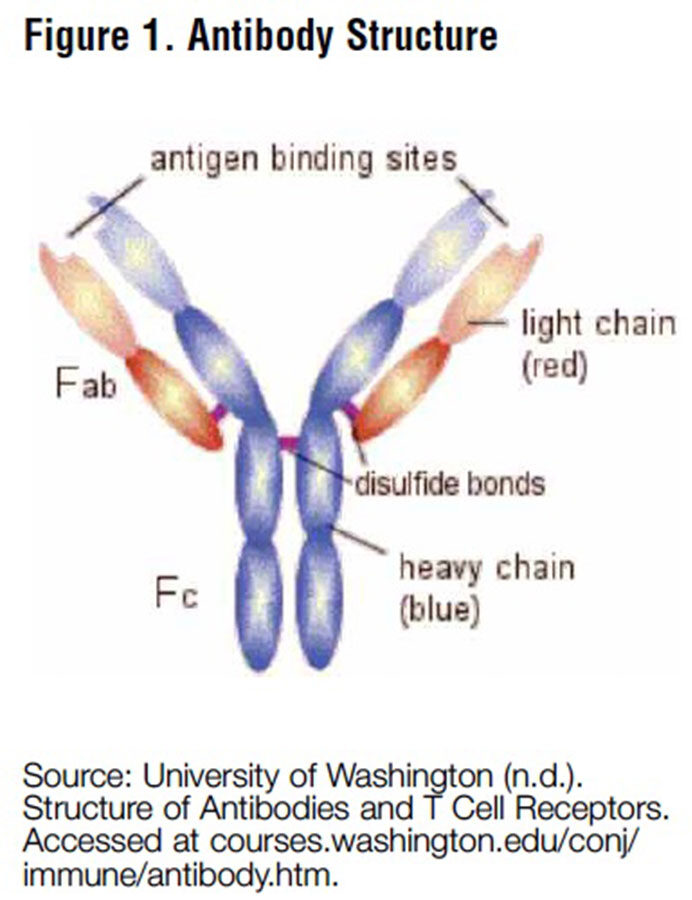
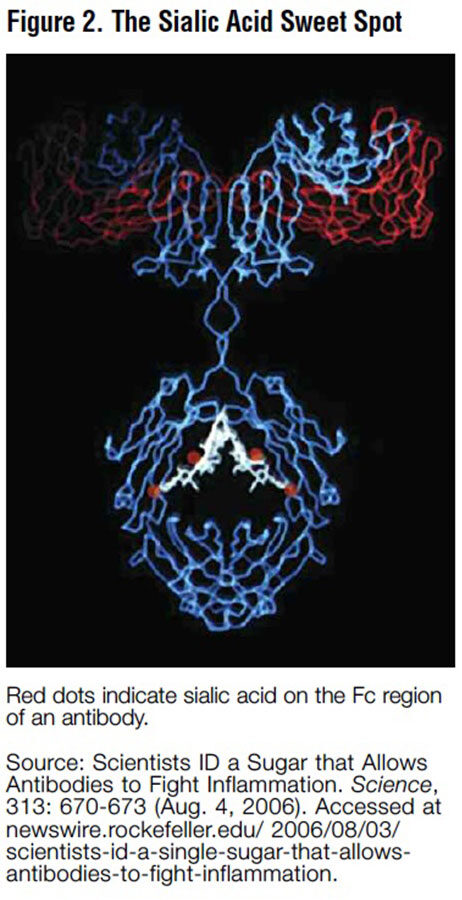
As shown in Figure 1, antibodies are Yshaped molecules. The stem of the Y is referred to as the Fc region, or heavy chain, which activates the Fc receptors involved in immune response. These Fc receptors appear to have a far greater affinity for the antibodies that have a sialic acid group attached to the Fc region, and when these sialylated anti- bodies activate the Fc receptors, the inflammatory response ceases (see Figure 2).2 Prior to this discovery, Dr. Ravetch and his team discovered that antibodies without a sialic acid group might be pathogenic because they actually promote autoimmune disease in mice.3
Dr. Ravetch and his team are now faced with how to turn this knowledge into more effective treatments for autoimmune disease. It has previously been demonstrated that enriching IVIG with sialic acid-linked antibodies results in a greater anti-inflammatory response.4 Even more exciting is that Dr. Ravetch and his team are able to create recombinant (laboratory made) sialylated Fc antibody regions that show a similar enhanced anti-inflammatory response.5 This means that autoimmune diseases could be treated more effectively at smaller doses using “sialic-switch” technology — either sialic acid-enriched IVIG or a drug that makes use of recombinant sialylated Fc antibody regions.5 Additionally, the use of a laboratory made molecule instead of a plasma-derived antibody could reduce dependence on plasma supply and even result in less frequent drug shortages.
Commercial Development
Sialic switch technology has been licensed to Momenta Pharmaceuticals in hopes of it commercializing an enhanced autoimmune disease treatment. While Momenta intends to continue to study the potential benefits of sialic acid-enhanced IVIG, it is looking to add recombinant products using the technology to their product pipeline in the near future.6
There’s no denying that IVIG has enhanced and even saved the lives of many autoimmune disease patients. But, the cost of therapy, large dose size and limited raw materials are serious limitations to an otherwise efficacious, well-tolerated therapy. If Momenta succeeds in exploiting the sialic switch technology, those with autoimmune disease could benefit from improved therapies within just a few years.
References
- Jolles S, Sewell WAC and Misbah SA. Clinical Uses of Intravenous Immunoglobulin. Clinical and Experimental Immunology, 142(1), 1-11.
- Anthony RM, Wermeling F, Karllson MC and Ravetch JV. Identification of a Receptor Required for the Anti-Inflammatory Activity of IVIG. Proceedings of the National Academy of Sciences, 105(50), 19571-19578.
- Nimmerjahn F, Anthony RM and Ravetch JV. Agalactosylated IgG Antibodies Depend on Cellular Fc Receptors for in Vivo Activity. Proceedings of the National Academy of Sciences, 104(20), 8433-8437.
- Kaneko Y, Nimmerjahn F and Ravetch JV. Scientists ID a Sugar that Allows Antibodies to Fight Inflammation. Science, 313, 670-673 (Aug. 4, 2006).
- Anthony RM, et al. (2008) Recapitulation of IVIG Anti-Inflammatory Activity with a Recombinant IgG Fc. Science, 2008 April 18; 320(5874):373-6.
- Momenta Pharmaceuticals (2014). Novel Autoimmune Drugs. Accessed at www.momentapharma.com/pipeline/sialylation-technology.php.