BabyBIG: Definitive Early Immunotherapy for Infant Botulism
Behind this highly effective public service orphan drug is a remarkable story of innovation, faith and perseverance.
- By Keith Berman, MPH, MBA
BOTULINUM TOXIN IS produced by the spore-forming, anaerobic bacterium Clostridium botulinum or other closely related bacterial species. It is the most poisonous substance known, natural or synthetic. The median lethal dose in adults is around one to two ten millionths of a gram when injected, or roughly 10 times that much when inhaled.1 Once carried through the bloodstream to the peripheral neuromuscular synapses, botulinum toxin blocks release of the acetylcholine neurotransmitter to muscle, resulting in hypotonia and flaccid paralysis. Without aggressive, around-the-clock medical support, death from a lethal exposure to botulinum toxin usually results from airway obstruction due to paralysis of pharyngeal muscles that control swallowing and airway diameter.
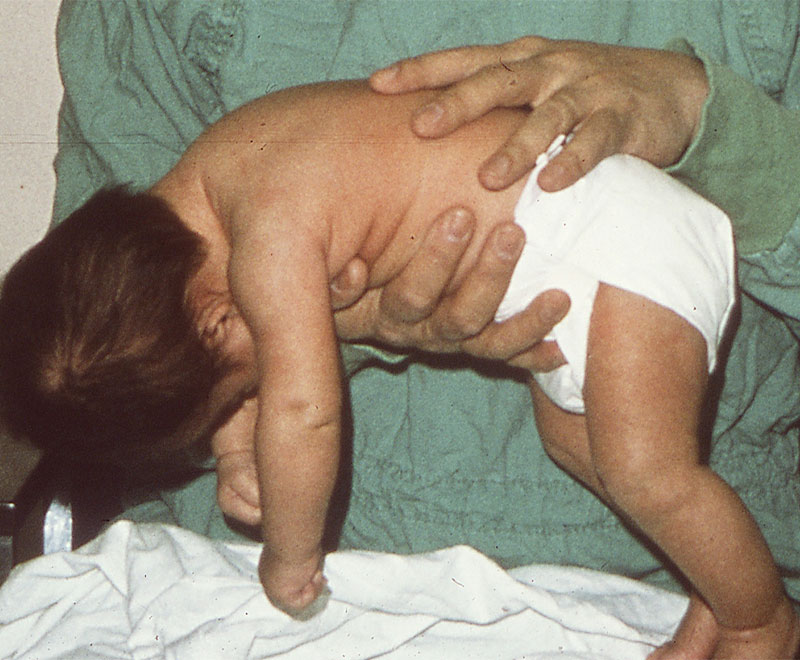
The ultimate survivors, spores of C. botulinum can reside dormant for years in soil, dust and other unfriendly environments. The spores germinate, multiply and start to release the botulinum neurotoxin only when they find themselves in suitable anaerobic (oxygen-free) conditions combined with an adequate nutrient supply. Over the 150 years since the first complete description of the symptoms of victims of a “sausage poisoning” outbreak around 1820, the acute paralytic disorder known as botulism* referred only to the sequelae of ingesting botulinum toxin present in food in which C. botulinum had grown.
Modern food preservation and processing methods variously kill the spores, inhibit growth of the organism or inactivate the neurotoxin. As a result, foodborne botulism is an extremely rare event today in the U.S., with fewer than 30 instances reported annually.2
A New Host for an Old Disease
However, in 1976, a pair of case reports described six very young U.S. infants with symptoms consistent with botulism that prominently included weakness and descending flaccid paralysis.3,4 All presented with an additional symptom: constipation. Yet none of them had any known or suspected exposure to foodborne botulinum toxin. Testing of fecal samples by Thaddeus Midura, PhD, Stephen Arnon, MD, and colleagues at what is now the California Department of Public Health (CDPH) solved the mystery: both C. botulinum bacteria and botulinum neurotoxin were found in the stools of all six infants.
Infant botulism was immediately recognized as an entirely new disease pathway. It occurs in susceptible weeks-to months-old infants who swallow a few spores of C. botulinum. The intestinal tract of these young infants, who are still consuming only a very simple diet of breast milk or formula, lacks the adult-type inhibitory bacterial flora that arrive once the baby starts solid foods. In the absence of those inhibitory bacteria that would otherwise hold C. botulinum spores in check, the gut of the infected infant is, in essence, an incubator that allows the spores to germinate, propagate and secrete botulinum toxin.
Although infant botulism was newly recognized in 1976, the disease itself was not new. Earlier misclassified cases were identified on retrospective reviews, including one laboratory- confirmed case dating back to 1931.5 Over the years prior to 1976, cases had simply been improperly attributed to some other disease process. Less than five years after the initial case reports, an epidemiological study conducted in California, where approximately half of U.S. cases are reported, found that about one in every 20 deaths reported as sudden infant death syndrome (SIDS) is actually caused by fulminant infant botulism.6
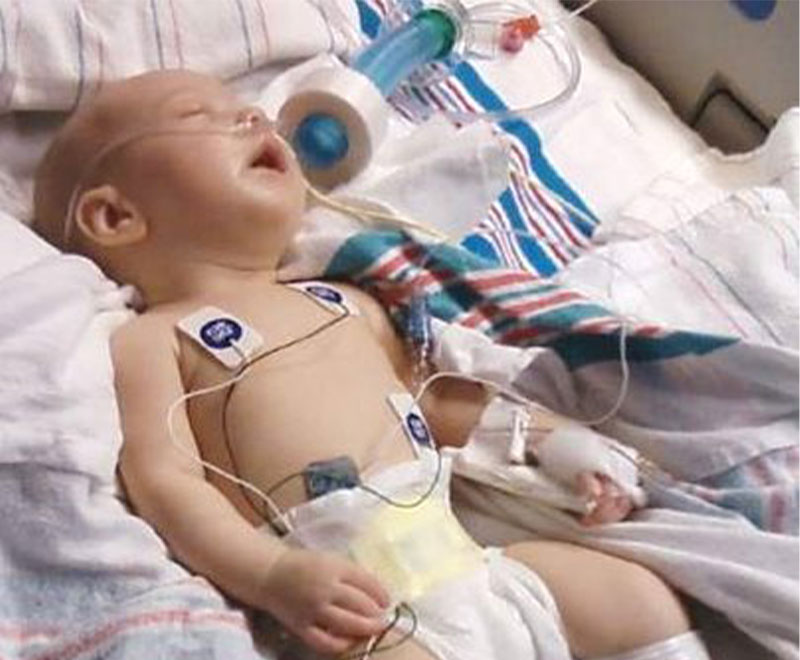
Over the ensuing 40 years, several thousand U.S. cases of infant botulism have been confirmed by fecal specimen testing. About 90 percent of the 100 to 150 cases reported annually occur in infants under 6 months of age; virtually all occur in babies under 1 year of age. In addition to California, case rates are much higher in a few other states, including Pennsylvania, Utah, Delaware and Hawaii. Soils in certain locations in those states have been found to contain C. botulinum spores that manage to find their way into microscopic dust and honey, which parents are warned not to feed to their children prior to 1 year of age.
With timely clinical diagnosis (Table 1) and 24-hour intensive supportive care, nearly all affected infants survive and eventually fully recover. But the potency and unusually long duration of action of botulinum toxin7 prolongs the neuromuscular blockade, resulting in a disease course that is lengthy and potentially complicated by such serious adverse events as pneumonia, anemia, hyponatremia and urinary tract infection. Roughly half of infants will require mechanical ventilation at some point during their hospital stay; the average period of ventilator dependency for those who do is about four weeks. While highly variable depending on patient-specific factors and whether affected by type A or type B botulinum toxin, untreated infant botulism patients remain hospitalized for close to six weeks on average with supportive medical care alone.

Creating a Botulism Immune Globulin
An equine botulism antitoxin, first used in the 1960s to hasten the recovery of adult patients with severe foodborne botulism, was ruled out for infant botulism. Its risks of serious side effects, including serum sickness, anaphylaxis and lifelong sensitization to equine proteins, outweighed the presumptive benefits of neutralizing the botulinum toxin. A CDPH team led by Dr. Arnon decided to investigate a different approach: administration of an antitoxin purified from the plasma of toxoid-immunized human donors. Thus began a 15-year odyssey (Table 2) that required Dr. Arnon and his colleagues to manage unforeseeable setbacks, navigate through a web of evolving laws and regulations, and enlist what amounted to an alphabet soup of federal and state government entities and altruistic volunteer plasma donors to produce and clinically test an experimental human botulinum antitoxin.
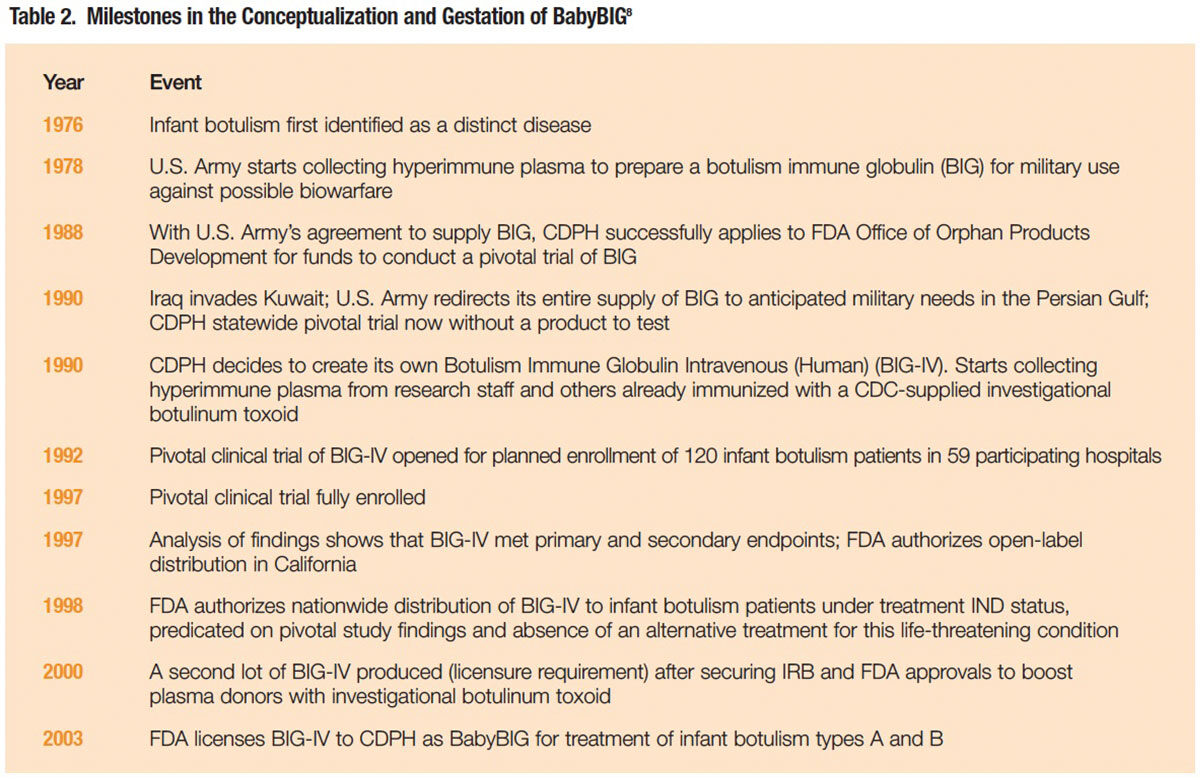
The initiative began with a major setback. In August 1990, just as organizational efforts were nearing completion for a randomized, placebo-controlled pivotal clinical trial of botulism immune globulin (BIG) to be supplied by the U.S. Army, Iraq invaded Kuwait. The Army redirected its entire supply of BIG to anticipated military needs in the Persian Gulf, and suddenly there was nothing to test.8
Not dissuaded, the CDPH, with support from the U.S. Food and Drug Administration (FDA) Orphan Drug Office, decided to create its own product to replace the Army’s diverted BIG product. For a source of hyperimmune plasma, the CDPH relied on volunteer plasma donations from its own botulism research staff and others previously immunized with a botulinum toxoid product for occupational safety purposes. The product prepared from that plasma was dubbed BIG-IV.
The pivotal trial to evaluate BIG-IV in 59 participating California hospitals finally opened for patient enrollment in February 1992. Over the next five years, 122 infants with laboratory-confirmed infant botulism were randomized to receive a single dose of BIG-IV or placebo. As the antibody has a half-life of approximately 28 days and a large capacity to neutralize botulinum toxin, a single infusion was demonstrated to be sufficient to neutralize, for at least six months, all of the neurotoxin that might be absorbed from the infant’s colon.
BIG-IV Proves Safe and Highly Effective
In May 1997, the study findings were unveiled. The mean length of hospital stay — the study’s primary endpoint — was significantly reduced in patients given a single dose of BIG-IV (2.6 versus 5.7 weeks, P<0.001). Essentially all of this reduction was reflected in a shorter mean intensive care unit (ICU) stay (1.8 versus 5.0 weeks, P<0.001). The mean duration of mechanical ventilation for the 59 patients requiring it also strongly favored the BIG-IV group (1.8 versus 0.4 weeks, P<0.001), as did duration of tube or IV feeding (3.6 versus 10.0 weeks, P<0.001).9
Earlier clinical diagnosis and administration of BIG-IV** directly translate into shortened hospitalization. The mean length of stay for patients administered BIG-IV within three days of admission was 2.0 weeks, compared with a mean stay of 2.9 weeks when given on days four to seven following admission (P<0.001).
Not surprisingly, the adverse event rate was reduced by nearly one-half in the BIG-IV group (0.9 versus 1.7 events, P<0.04). Anemia and urinary tract infection rates were significantly lower, with rates of other serious adverse events trending lower as well.
All of these clinical benefits translated into mean hospital charges per patient of $74,800 in the BIG-IV group, 54 percent lower than mean charges of $163,400 in the placebo group.
Finally in October 2003, an effort that began in 1988 with plans to test an Army BIG product culminated in the FDA’s approval of Botulism Immune Globulin Intravenous (Human) (BIG-IV), trade named BabyBIG. The product license is held by the CDPH Infant Botulism Treatment and Prevention Program (IBTPP). BabyBIG is manufactured by Baxalta on a contract basis. It is distributed at the direction of IBTPP to hospitals throughout the U.S. and internationally by FFF Enterprises. As a not-for-profit, self-supporting state activity, IBTPP uses revenues eventually paid by health insurers for the product to fund its operations, including laboratory testing and 24-hour on-call availability of a physician botulism treatment specialist (see How to Contact the Infant Botulism Treatment and Prevention Program).
The Rewards of Staying the Course
Since IBTPP began supplying BabyBIG, it has carefully tracked patient outcomes and hospital charges, and documented that the product has spared infants more than 70 years of hospitalization (mostly in the ICU) and has reduced hospital charges by more than $100 million.
Over a period of 15 years, BabyBIG was produced, clinically tested and licensed with a cash outlay of just $10.6 million (in 2005 dollars).8 That shoestring budget does not, however, account for countless thousands of hours devoted by personnel at the CDPH and other collaborating agencies to make it all happen.
“In my younger days, my avocation was mountain climbing,” Dr. Arnon shared with me in an interview. “Which, by definition, is always going uphill. In its own way, it was good preparation for the BabyBIG endeavor.” As a result of the perseverance and inventiveness of this state public health department, somewhere every few days a young infant paralyzed by the world’s most potent toxin is discharged home weeks earlier and with a significantly lower risk of serious complications than he or she would have otherwise without BabyBIG treatment.
Without question, it was a mountain worth the climb.

* From the Latin word “botulus,” meaning sausage.
** As currently recommended for all new patients with a provisional clinical diagnosis, treatment with BIG-IV was started as early in the illness as possible to maximally neutralize the toxemia; it should not be delayed for laboratory confirmation of the clinical diagnosis.
References
- Arnon SS, Schechter R, Inglesby TV, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA 2001 Feb 28;285(8):1059-70.
- www.cdc/gov/nczved/divisions/dfbmd/diseases/botulism. Accessed 10/30/2015.
- Pickett J, Berg B, Chaplin E, et al. Syndrome of botulism in infancy: clinical and electrophysiologic study. New Engl J Med 1976 Sep 30;295:770-2.
- Midura F and Arnon SS. Identification of Clostridium botulinum and its toxins in faeces. Lancet 1976 Oct 30;2(7992):934-6.
- Arnon SS, Werner SB, Faber HK, et al. Infant botulism in 1931: discovery of a misclassified case. Am J Dis Child 1979 Jun;133(6):580-2.
- Arnon SS, Damas K, Chin J. Infant botulism: epidemiology and relation to sudden infant death syndrome. Epidem Rev 1981;3:45-66.
- Ravichandran E, Gong Y, Al Saleem FH, et al. An initial assessment of the systemic pharmacokinetics of botulinum toxin. J Pharmacol 2006 Sep;318(3):1343-51.
- Arnon SS. Creation and development of the public health service orphan drug human botulism immune globulin. Pediatrics 2007 Apr;119(4):785-9.
- Arnon SS, Schechter R, Maslanka SE, et al. Human botulism immune globulin for the treatment of infant botulism. New Engl J Med 2006 Feb 2;354(5):462-71.
- Mitchell WG, Tseng-Ong L. Catastrophic presentation of infant botulism may obscure or delay diagnosis. Pediatrics 2005 Sep;116(3):e436-8.