Biosimilars Debut in the U.S.
- By BSTQ Staff
In light of the angst and hand-wringing over the soaring costs of biologic drugs that are alleged to be contributing to the unsustainability of biologic treatments, the U.S. Food and Drug Administration (FDA) approval of the first biosimilar in the U.S. was welcome news to many. A biosimilar product is one that has shown it is highly similar to an already approved biological product, known as a reference product. In addition to similarity, a biosimilar must show it has no clinically meaningful differences in terms of safety and effectiveness from the reference product. Only minor differences in clinically inactive components are allowable.
Practices that understand this class of products, make definitive preparations and understand the payers’ options for using them can potentially benefit their patients and their bottom line. But changes in patient and provider education programs will most likely be needed.
The Biosimilar Distinction
Biosimilars are distinct from generics. The active ingredients of generics are identical to the small molecule reference products because generic manufacturers can follow a specific formulation or recipe. This is not possible with biologic products that tend to have very large molecule structures leading to much higher development complexity for biosimilars, which renders biosimilars unique to each manufacturer and cell line.
In 2013, the top-eight best-selling biologics accounted for almost $63 billion in sales. These included Humira, Remicade, Rituxan, Enbrel, Lantus, Avastin, Herceptin and Neulasta. Lantus’s patent expired in 2014; Neulasta’s will expire in 2015. Humira’s and Rituxan’s will expire in 2016; Remicade’s will expire in 2018; Avastin’s and Herceptin’s will expire in 2019; and Enbrel’s will expire in 2028.1
Just as generic drugs introduced to market upon the expiration of the branded product brought choice of a less-expensive alternative, so too will biosimilar products introduced to market upon the expiration of the branded biologic product. But unlike generics, biosimilars will likely be priced at only a 20 percent to 40 percent discount compared with reference biologics. This is because biosimilar development is much more complex and costly largely due to the fact that they require far more clinical and nonclinical testing and a longer development period. Still, this is a significant potential savings given the total costs associated with biologics.
Biosimilars submitted to FDA for approval may be newly minted biologic products developed by U.S.-based pharmaceutical companies, or they may be biologic products already approved and used in other countries. Interestingly, the long-awaited U.S. move to ICD-10 classifications for diseases will make use of this comparative data considerably easier, since the rest of the world has been using this classification for many years.
The First U.S. Biosimilar
The first and only (at this time) biosimilar product in the U.S. — Zarxio (filgrastim-sndz) for the treatment of infection in certain cancer patients undergoing chemotherapy — was approved by FDA on March 6. This product is a biosimilar version of Amgen Inc.’s Neupogen (filgrastim) and is the first U.S. biosimilar approved through an accelerated pathway authorized by the Affordable Care Act (ACA). The ACA amends the Public Health Service Act to create an abbreviated licensure pathway for biological products that are demonstrated to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product.
At this early point, the actual market arrival and initial cost, as well as the final name for Zarxio (filgrastim-sndz), remain unclear. As of now, the “filgrastim” component defines the biologic and “sndz” defines the manufacturer. But two other decisions still need to be made by FDA: whether biosimilars can share the same nonproprietary names as their reference drugs, and what data FDA needs to determine that a biosimilar can be deemed interchangeable. The latter will take into account whether there are subtle differences with the terms “biosimilarity” and “interchangeability.” This designation will determine the steps a pharmacy needs to take to fill the prescription or provide the drug upon receipt of a physician order. State regulations also play a role, and several are adding guidance to their regulations.
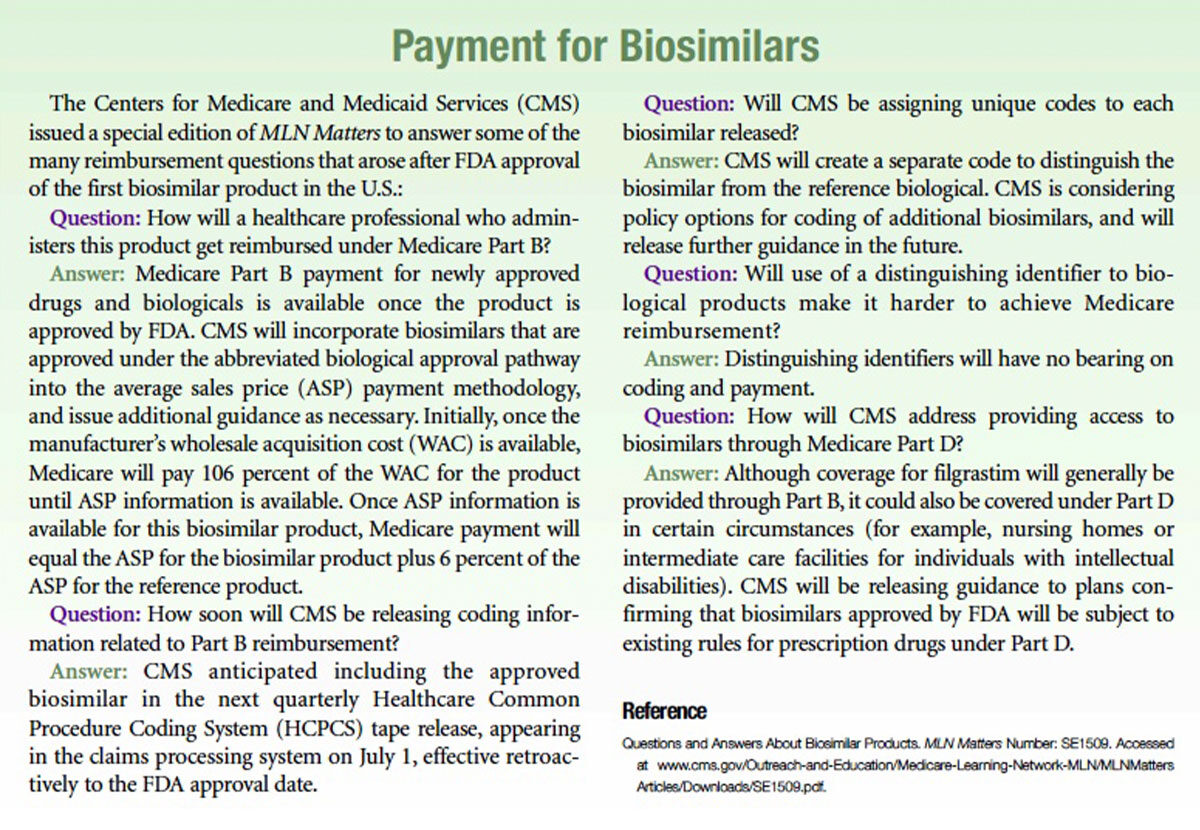
References
- Biologics Still on Top in Best-Selling Drugs in 2013. The Cell Culture Dish, March 13, 2014. Accessed at cellculturedish.com/2014/03/top-ten-biologics-2013-us-pharmaceuticalsales-2.