How Proposed 2018 OPPS Payment Rules Impact Pharmacy
- By Bonnie Kirschenbaum, MS, FASHP, FCSHP
THE OUTPATIENT Prospective Payment System (OPPS) determines how the Centers for Medicare and Medicaid Services (CMS) pays for Medicare patients treated in outpatient settings. For 2018, the proposed changes to the OPPS rules have been published in the Federal Register, the comment period has ended, and the final rules will be decided before the year’s end. The impact of changes to the rules is broad since the scope of “outpatient” includes a vast array of clinics, infusion centers, treatment and diagnostic areas and emergency rooms. Payment for drugs, biologics and radiopharmaceuticals in these areas is covered under the Medicare Part B medical benefit. It’s important to differentiate these from 1) drugs used in the ambulatory setting that fall under Medicare Part D and are part of the pharmacy benefit, and 2) those not billable because they are considered self-administered.
This column addresses how payment for drugs and biologics works, the effect Medicare decisions have on private payers and the proposed drastic reduction in payments for 340B drugs in settings covered by OPPS.
CMS Payment for Part B Drugs Under OPPS
CMS pays for Part B drugs in five different ways divided into two categories: those that are separately payable and those that are bundled or packaged.
Drugs that are separately payable have line-item reimbursement. These are:
- New drugs not yet assigned a unique Healthcare Common Procedure Coding System (HCPCS) code (no proposed change)
- New pass-through drugs, biologics and radiopharmaceuticals (several proposed changes)
- Specified covered outpatient drugs (proposed threshold change) Payable drugs with no line-item reimbursement because they are bundled or packaged are:
- Lower-cost packaged products costing less than $120 per day (proposed threshold up from $110 per day in 2017)
- Products used in policy packaged services, regardless of cost (no proposed change)
Payment for all policy packaged drugs, biologics and radiopharmaceuticals is included in the services and procedures with which they are reported. These are non-pass-through drugs, biologics and radiopharmaceuticals, including:
- All diagnostic radiopharmaceuticals
- All contrast agents
- Anesthesia drugs
- Implantable biologics that are surgically inserted or implanted in the body through a surgical incision or natural orifice
- Drugs, biologics and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure
- Drugs and biologics that function as supplies or implantable devices in a surgical procedure
Regardless of which category a drug falls into, every drug must be billed. Neglecting to bill will automatically cancel a request for payment for drug administration fees. It will also report inaccurate data to the payer and the big data pool, which will lead to artificially lowered reimbursement for bundles/ packages in subsequent years.
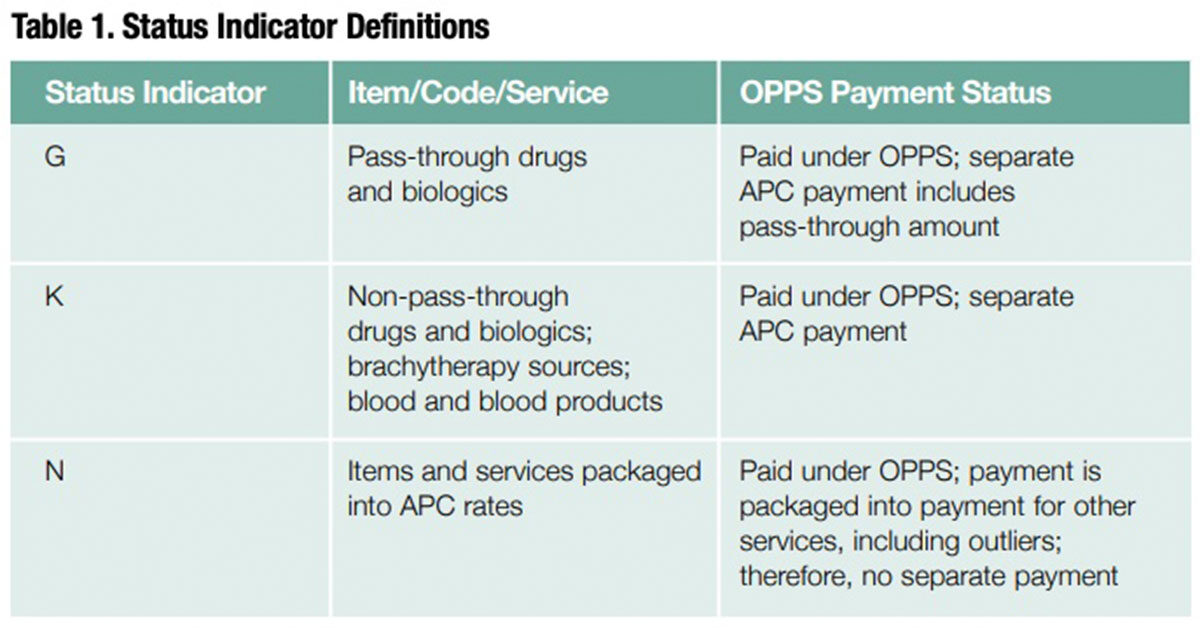
Proposed 2018 Changes for Separately Payable Drugs
Transitional pass-through/non-passthrough separately payable drugs are currently paid at average sales prices (ASP) plus 6% minus 2% sequestration. These drugs move from annual to quarterly pass-through expiration status for devices, drugs and biologics ending in the quarter that is as close to three full years as possible after the products were first covered with a pass-through payment. The 2018 proposal lists 38 drugs with new/continuing pass-through status and 15 that lose pass-through status and move from status indicator (SI) G (pass-through) to SI K (separately payable). Four drugs lose pass-through status and move from SI G (passthrough) to SI N (items and services packaged into ambulatory payment classification [APC] rates).
Drugs and biologics are first eligible for pass-through status the quarter following application approval, and CMS will calculate the offset amount for passthrough payments at the HCPCS code level rather than the APC code level.
Drugs and biologics that are separately payable will continue to be paid at ASP plus 6% minus 2% sequestration under the statutory default payment policy adopted in 2013. CMS will pay all non-pass-through separately payable therapeutic radiopharmaceuticals at ASP plus 6% minus 2% sequestration as well. However, radiopharmaceutical manufacturers are not required to submit ASP. While some manufacturers voluntarily submit ASP, CMS will use that for a “patient-ready” dose. If ASP is not available, CMS will base payment on mean unit cost from its claims data.
To prepare for these proposed changes, pharmacy providers should work with their revenue cycle team to ensure all drugs with SIs G, K and N are billed regardless of whether they are separately payable. They should also update their list of waste billing drugs. And, they should remove the four pass-through drugs that are moving from SI G to SI N, as well as lower-priced drugs that no longer meet the threshold and will move from SI K to SI N and will no longer be eligible for waste billing as of Jan. 1, 2018. Table 1 provides a list of SI definitions.
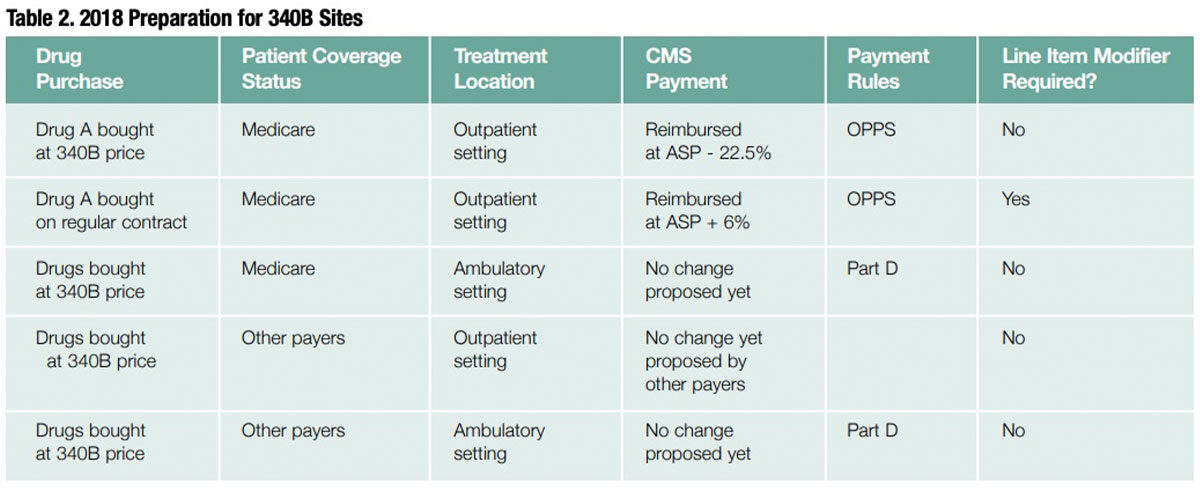
Proposed Payment Rate Changes for Certain Medicare Part B Drugs Purchased by Hospitals Through the 340B Program
For certain Medicare Part B 340B program drugs, the proposal is to cut reimbursement for 340B facilities from ASP plus 6% to ASP minus 22.5% minus 2% sequestration. CMS also is proposing that a modifier be added to each line item billed to a Medicare patient in an OPPS setting when a 340B drug is being used so it can identify which drugs will be reimbursed at that rate. This proposal is based on MedPac recommendations, and they apply only to Medicare patients treated in an OPPS setting. Others may adopt them as time goes on, but this is the limit as of now. CMS is requesting comments on how it can best implement this proposal to pass savings on to beneficiaries and providers, and to allow seniors to save money on drug costs. See Table 2.
Limited to Separately Payable Drugs
Only separately payable drugs are affected by this proposed ruling and only when used for Medicare in outpatient settings. According to CMS, “We believe that any payment changes we adopt should be limited to separately payable drugs under the OPPS, with other additional exclusions. These exclusions include 1) drugs on pass-through status, which are required to be paid based on the ASP methodology, and 2) vaccines, which are excluded from the 340B program.” In addition, CMS is soliciting comments on whether other types of drugs such as blood clotting factors should be excluded from the reduced payment.