ICD-10, Audits and Authorizations
- By Bonnie Kirschenbaum, MS, FASHP, FCSHP
Healthcare practitioners may feel overwhelmed amid the onslaught of payment reforms brought about by unsustainable increasing healthcare costs. A substantial portion of these changes is related to drugs and biologicals, including immunotherapy agents. The interrelationship between three major issues — ICD-10 conversion, the increasing burden of recovery auditor contractor (RAC) audits and the need to streamline authorizations and meet local coverage determination (LCD) and national coverage determination (NCD) requirements — that all practices and facilities are grappling with presents an interesting opportunity. Rather than being overwhelmed by the perceived complexity of solving three complicated issues, practitioners can learn to be cross-functional by recognizing how solutions to one issue can depend upon and assist with solutions to the other two.
ICD Conversion
Created years ago initially as a mechanism for categorizing death during times of war, the International Classification of Diseases, or ICD (now the responsibility of the World Health Organization), is used to standardize codes for medical conditions and procedures. Standardization allows the world to use a common language to compare and share health information. Even though most countries already use the 10th revision of these codes (ICD-10), the United States is still in the process of adopting it, and it’s the last major industrialized nation to do so. ICD-9 codes are now over 35 years old, have a very limited number of new codes that can be created since categories are too full for expansion, and don’t accurately represent current detailed specific disease state descriptions and definitions. ICD-9 codes are also inconsistent with current medical practice — so much so that several ICD-9 codes often have to be combined or modified to accurately represent the complexity of the patient. The ICD-10 code set has thousands more codes to choose from to achieve a much greater level of specificity. And, therein lies one of the problems with the transition: It’s not just a simple crosswalk; instead, it will require practitioners to completely relearn how disease states are coded.
Healthcare practitioners had to begin using the ICD-10 codes on Oct. 1. Codes are assigned by revenue cycle team coders who review what is in patients’ medical records to find disease states and conditions along with procedures and treatments. For the new code set to succeed, the documentation in electronic health records (EHRs) and the modification of order sets in computerized physician order entries (CPOEs) need to be substantially improved in both the inpatient and outpatient settings. If coders cannot find documentation of the complexity of patients’ conditions and subsequent treatment plans, they will not be able to code appropriately using the new ICD-10 code set. Although the current procedural terminology and/or healthcare common procedure coding system payment codes do not change either in their descriptions or their rates, the link between these codes and the correct ICD-10 diagnosis codes is essential to ensure payment. The onus definitely is on clinicians to significantly improve their documentation.
RAC Audits
The increasing burden of RAC audits affects healthcare facilities both administratively and financially. Currently, most RAC audits are for medical necessity issues. This means practitioners must be cognizant of LCD and NCD requirements or prior authorizations for an ever-increasing number of drugs, biologics and immunotherapy agents. It also means that documentation is essential. For instance, when the payer receives a claim for a treatment or drug and the substantiating information that supports that claim has not been sent along with it, that translates to poor documentation. If there is nothing in the medical record for the coders to code, nothing can be sent to the payer.
These audits are designed to ensure that the drug chosen is appropriate for the condition the patient is being treated for. Auditors often rely on published national guidelines (e.g., National Comprehensive Cancer Network guidelines for oncology patients) or on well-documented pathways of treatment. In essence, they link the drug chosen with a diagnosis and the procedures performed. But all those diagnosis codes are about to change. This is why practitioners must be prepared and involved in the process as they transition to ICD-10. They must play a role in planningfor possible contingencies related to denied or delayed claims that affect their drug budget.
Streamlining Authorizations
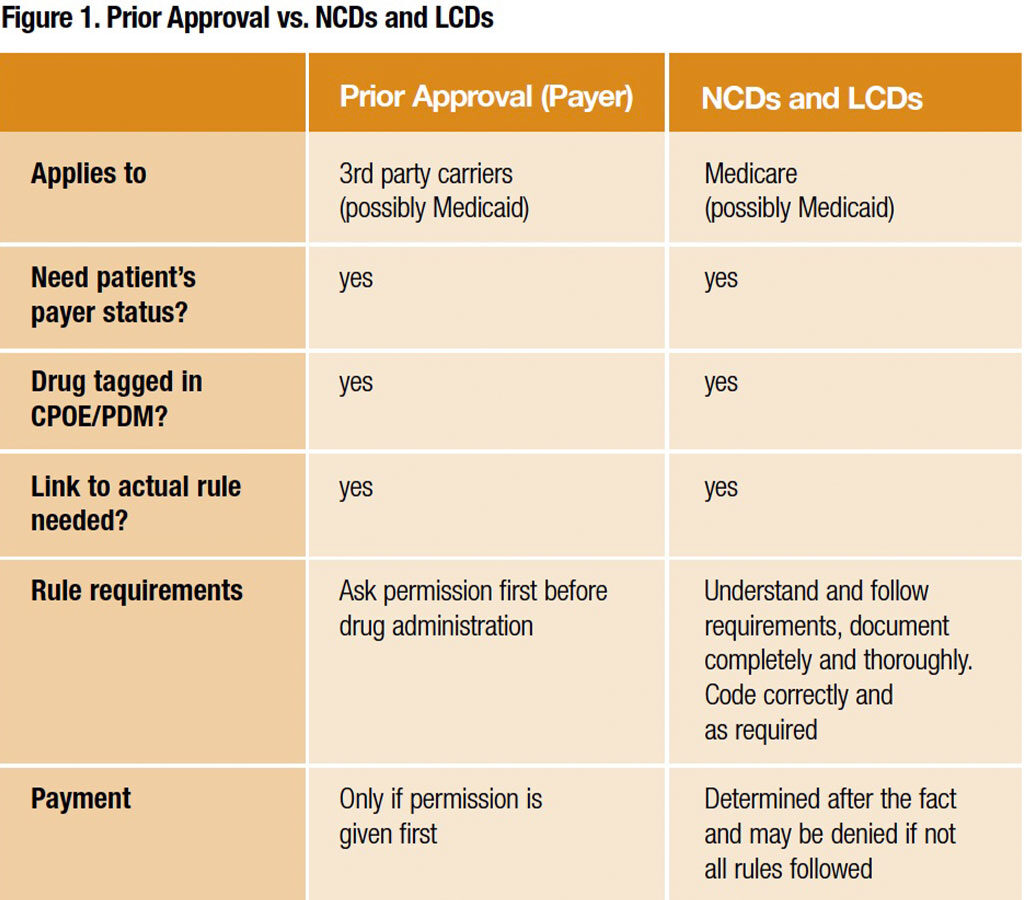
The need to streamline prior authorizations and meet LCD and NCD requirements to ensure payment for drugs is essential. Figure 1 suggests how using existing systems can substantially improve this process.
Following are a couple of suggestions to ensure authorizations are streamlined: 1) Prepare a list of all drugs with LCD and NCD or prior authorization requirements; correct linkage to the new ICD-10 codes will determine payment for them. Pick one or two drugs to use as an example of impact. Although a patient navigator or other individuals may be responsible for actually seeking and fulfilling these requirements, it’s the practitioner’s budget that will be negatively affected if payment is denied or delayed. 2) Review all medication orders in the CPOE system that are connected to a particular disease state or defined pathway; correct linkage to the new ICD-10 codes is essential. Pick a few order sets to use as an example of impact, and review all clinical trials the practice is involved in because documentation of patient eligibility will be changed with the new codes.
Common Ground
What do these three issues — ICD-10 transition, audits and authorizations — share in common? They use the substantially improved complexities and descriptions in the ICD-10 codes to document why a patient is being treated and for which conditions, along with meeting the requirements posed by prior authorizations, LCDs and NCDs to ensure accurate payment and get rid of RACs! It’s all about documentation. What’s more is that the new ICD-10 codes compare patients to those with worldwide access to the biosimilar drugs and biologics that will be making inroads in the U.S.