Revisiting Billing for Expensive Drug Waste
- By Bonnie Kirschenbaum, MS, FASHP, FCSHP
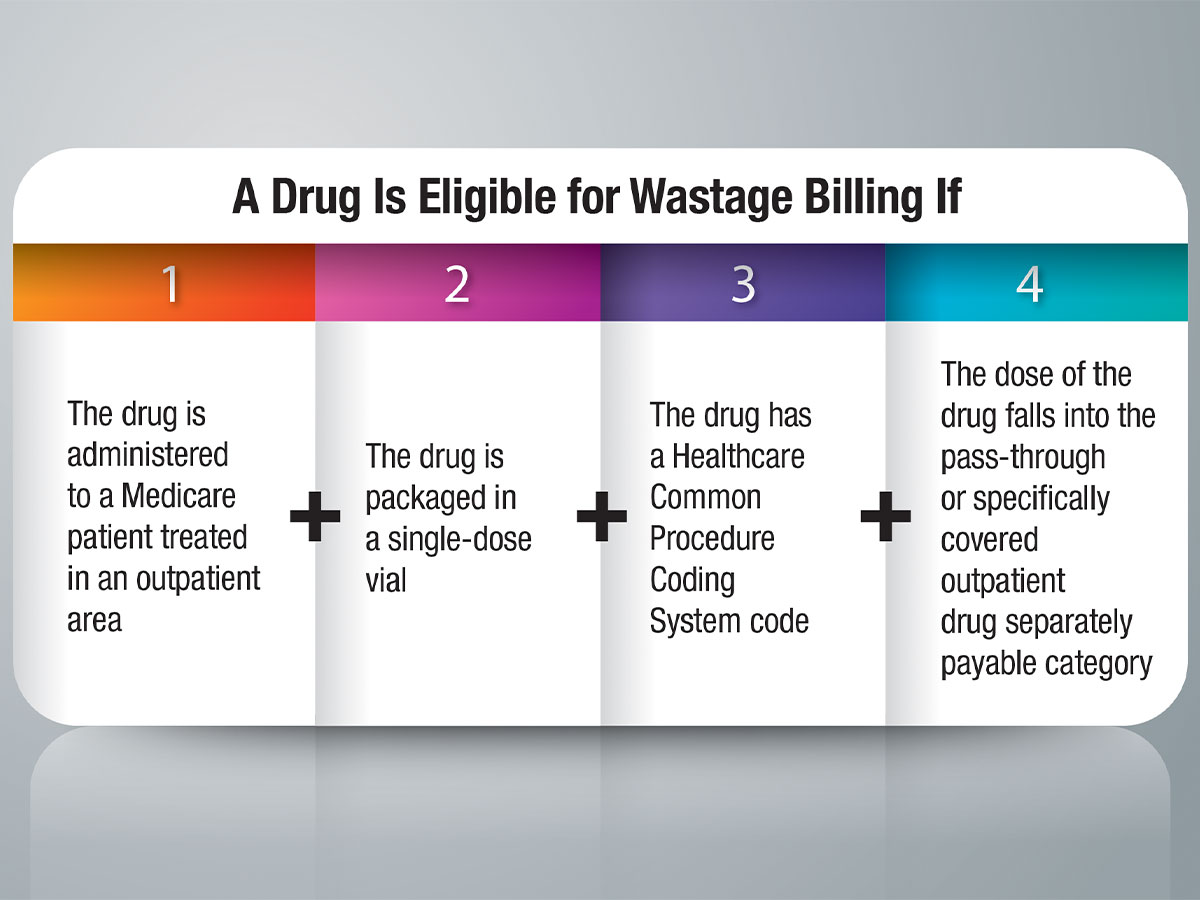
ALTHOUGH BILLING FOR drug waste sounds simple, confusion reigns for many healthcare providers, thwarting this revenue stream at many facilities. A simple test to determine whether to bill for an unused portion of drug can be answered with four questions: 1) Is the drug administered to a Medicare patient treated in an outpatient area? 2) Is the drug packaged in a single-dose vial? 3) Does the drug have a Healthcare Common Procedure Coding System code? 4) Does the dose fall into the pass-through or specifically covered outpatient drug separately payable category (versus the less-than-$120-per-day bundle or any other packaged or bundled payment category)? If the answer to all four questions is yes, drug waste should be billed. If the answer to any of these questions is no, drug waste is not billable.
Historical Perspective on Drug Waste Billing
Some of the mystery surrounding drug waste billing can be solved by understanding the payment rule history. In 2004, under the Outpatient Prospective Payment System (OPPS), Medicare ceased paying for the whole vial of a drug and moved to reimbursement of “billing units representing actual dose given.” This was a financial shock to providers who then lobbied for compensation for wasted drug. In 2007, billing for expensive drug waste was introduced. Medicare doesn’t mandate billing for waste, but makes it possible to recoup some money if providers choose to bill for the lost drugs. To be sure, providers must pay strict attention to the OPPS rules. In fact, the Centers for Medicare and Medicaid Services (CMS) encourages patient scheduling to ensure drugs are used efficiently. If the remainder of a single-dose vial must be discarded after the drug is administered, the program covers both the amount administered and discarded.
Providers Lag in Drug Waste Billing
Unfortunately, many providers fail to bill for drug waste. Low implementation of waste billing is a result of a combination of factors, including knowledge gaps, a perceived low return, perceived resource constraints (too much complex work for the resulting yield), lack of IT systems support for the required level of automation and documentation in the outpatient area, a business risk assessment (the fear that a change in billing might lead to scrutiny) or some other set of circumstances.
Regardless of the rationale, the decision not to bill for outpatient drug waste is hurting everyone — especially now that bundled payment models are in vogue. Bundled payment calculations are based on “big data,” including the history of payment for separately payable medications and biologics, drug administration costs and payment for waste and the myriad non-separately reimbursable products used. The decision to not implement waste billing (or to not bill for non-separately reimbursable products) paints an inaccurately low picture of the true cost of medications being included in the bundled payment.
How to Bill for Drug Waste
Preparation for drug billing in outpatient clinics and treatment areas must match what’s billed and charted in the electronic health record. Here are the steps to implement and/or review when going forward with billing for drug waste:
1) Determine which drugs are going to be waste candidates. Each drug reimbursed by Medicare under OPPS has been assigned a status indicator (SI). Only those with SI G or SI K are eligible for waste billing, and only when a single dose vial, ampule and/or syringe is used. SI G indicates the drug has pass-through status and will be paid for separately as indicated by statute. SI K indicates the drug is a separately covered item that costs more than $120 per day as defined by the current 2018 OPPS rules. Simply looking at the drugs on your formulary and choosing only the ones with these two status indicators will produce a manageable first-draft listing of waste candidates. Further narrow this list by removing all products that are not in single-dose packaging. Next, select the products from this list that your outpatient departments will benefit most from waste billing. For example, you may decide to identify only a handful of expensive agents in the infusion clinic or specialty outpatient clinics for wastage billing.
2) Create a charge description number (CDM) for the selected drug and a corresponding pharmacy drug master description for the drug waste. For example, drug A CDM #123456 and drug A waste CDM# 123457. This allows a clear pathway to be created as part of drug order entry. The actual amount of the drug used will be entered, as well as the actual amount of the drug wasted. Both of these entries will appear on the medication administration record (as required), and both will proceed to billing on the same day (also as required). By using this dual drug order entry, you will have created the required documentation for both the dose of drug administered and the waste in the electronic medical record.
3) When determining drug waste, convert the dose administered into billing units, and round up to the next whole billing unit as needed. The balance of the drug from the single-dose vial will be considered waste, and should also be converted into billing units. This can be done automatically with a crosswalk built into your system, or it may be done manually with each order entry.
4) The revenue cycle team then needs to add the mandatory JW modifier code to the drug waste billed to differentiate it from the dose of the drug administered.
5) Develop policies and procedures for wastage billing, and orient your staff, including the nursing and revenue cycle teams. Stress that in the CMS world, you cannot ever bill for more than the total amount of drug in the single-dose vial.
Notes
- The Medicare Claims Processing Manual, Chapter 17, Section 40, provides policy detailing the use of the JW modifier for discarded Part B drugs and biologicals.
- The official instruction, CR9603, issued to Medicare Administrative Contractors regarding the JW modifier change is available at www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R3538CP.pdf.
- To access quarterly average sales price tables, go to www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2017ASPFiles.html. Focus on the ASP crosswalks(NDC to HCPCS).
