Saving the Aging Brain: Grifols Attacks Alzheimer’s Disease Head-On
- By Keith Berman, MPH, MBA
IT WAS OUR great fortune to have been born well into the 20th century. As recently as 1900, disease, poor nutrition and unsafe food and water limited U.S. life expectancy to less than 48 years. Since then, a myriad of 20th century advances in medicine, public health and sanitation have combined to dramatically extend average adult lifespan. People who reach 65 years of age can expect, on average, to live nearly 20 more years.1
But for an estimated five million Americans living with Alzheimer’s disease (AD), an unrelenting decline in cognition, memory and capacity for self-care makes the last years of life anything but a blessing. Millions of others experiencing nagging signs of mild cognitive impairment must face each day wondering if they are gradually descending into a dark hell that will eventually rob them of their minds and their dignity.
Most chronic age-associated conditions are highly treatable. When diagnosed at an early stage, many are preventable, reversible or even curable. None of these positive scenarios currently applies for AD. A diagnosis cannot be made until the neurodegenerative process has advanced over many years to the point where frank evidence of dementia is apparent and much irreversible damage is done. Notwithstanding certain lifestyle choices that might mitigate disease risk (e.g., diet and exercise), Alzheimer’s cannot be prevented; it will inevitably afflict one in nine persons over age 65 and one in three over age 85.2 And once diagnosed, physicians can offer palliative drugs that may temporarily help control some symptoms, but nothing in their armamentarium to slow progression of the disease.
Numerous academic and industry teams are investigating early diagnostic tests, vaccines to delay or prevent progression to full-blown dementia, or treatments for persons already affected with mild to moderate AD. For nearly a decade, Grifols, the world’s third largest manufacturer of plasma-based therapeutics and a leader in immunohematology and transfusion medicine diagnostics, has been patiently developing a novel approach to treatment of AD, based on plasma exchange and replacement with donor human albumin. Then on the eve of World Alzheimer’s Day in September 2012, the Barcelona-based company announced a bold new “global” AD research and development strategy. Together with majority-owned Araclon Biotech, Grifols has committed to an extraordinarily ambitious three-pronged attack on this disease: early diagnosis, prevention and treatment.
Early Diagnosis: The ABtests
A 2004 spinoff of the University of Zaragosa, Araclon Biotech is developing two patented sandwich ELISA colorimetric test kits, dubbed ABtest 40 and ABtest 42, for direct determination of amyloid-β 40 and 42 peptides in blood. While their precise role is not fully understood, these proteins have been clearly implicated in the pathophysiology of AD.
Many large multicenter initiatives have proposed elaborate models that exploit the most widely validated AD biomarkers — MRI, PIB-PET, FDGPET. and CSF levels of amyloid-β (Aβ), tau and phosphorylated-tau — to identify persons in early stages of AD. Unfortunately, this approach is hampered by practical considerations that severely limit their broad application. “The feasibility of these biomarkers for screening the general population once a preventive treatment has been developed also remains questionable,” according to Araclon scientists.
Both because of their comparative simplicity and accumulating evidence that changes in brain Aβ are among the first detectable signs of disease onset, interest has turned to blood-based biomarkers, and the Aβ 40 and 42 peptides in particular. But different studies have yielded inconsistent results,3,4 and variability in assay methods have been suggested as a reason.5 Another potential confounder of these analyses is the fact that conventional tests measure only the Aβ peptides found free in plasma, which may be as little as 15 percent of total blood Aβ; the rest is bound to albumin and other plasma proteins, as well as to red blood cells.
A unique feature of Araclon’s ABtest is its ability to quantify all Aβ peptide in blood — not just the free unbound portion in plasma. Using its battery of proprietary ABtest assays, the company recently identified four markers from the Aβ pool in blood that differed significantly between a group of MCI patients and a healthy control group, after adjusting for relevant demographic covariables.6 Araclon is currently conducting studies involving more than 400 individuals with the goal of validating its ABtest kits as screening tools for diagnosis of early pre-symptomatic AD.
Active Immunization as a Preventive
In 1999, scientists at Elan Pharmaceuticals reported that injections of Aβ into a transgenic mouse model of AD resulted in virtually complete clearance of amyloid-β plaques, instantly suggesting an exciting new therapeutic approach.7 The animals developed high titers of antibodies directed against Aβ, and in short order, Elan and Wyeth were at work on an Aβ vaccine. But the program was halted in 2002 when a few subjects experienced brain inflammation resembling aseptic meningoencephalitis, leading to the conclusion that the vaccine induced Aβ-reactive T cells that mediated widespread inflammation.8
More than a decade on, Araclon has readied a new and uniquely configured vaccine directed against Aβ1-40, the most abundant Aβ isoform in the brain and blood and known to form neurofibrillary tangle-like structures in persons with AD. Araclon scientists believe their ABvac40 vaccine, which consists of a short C-terminal fragment of Aβ1-40 cross-linked to a carrier protein and formulated with an alumhydroxide gel adjuvant, will be far less apt to induce unwanted collateral effects that plagued the old Elan vaccine. Toxicology studies in rats and rabbits have revealed an excellent safety profile for ABvac40, with no signs of cerebral inflammatory activity.
In September 2013, a Phase 1 safety and tolerability study was approved by the Spanish Medicines Agency, and enrollment of patients with mild to moderate AD began in 2014. Ideally, if all goes as planned, physicians will utilize ABtest assays to help diagnose incipient disease years before progression to the clinical stage, and actively immunize these individuals with ABvac40 to prevent or delay AD.
The path to approval of the ABvac40 vaccine will be long and, given our limited understanding of AD pathophysiology, there is substantial risk that this and other prospective vaccines will come up short. Grifols and Araclon are undaunted; they have confidence in their science, and they recognize the enormity of the need. For individuals with early-stage AD and their families, the importance of a vaccine that can stave off its advance simply cannot be overstated.
The Powerful Logic of Plasma Exchange to Treat AD
The therapeutic principle underpinning Grifols’ novel investigational AD treatment — essentially a modified regimen of plasmapheresis with albumin replacement — is elegantly simple and compelling. It is predicated on these observations:
- Available evidence suggests that certain Aβ peptides are neurotoxic; Aβ aggregates that accumulate as neuritic plaques are a histopathological hallmark of AD.
- It is now recognized that a chronic, mild pro-inflammatory state is correlated with the major degenerative diseases of the elderly, including AD.9
- Well-characterized mechanisms enable Aβ to cross from the circulation through the blood-brain barrier (BBB) into the brain, and to be cleared from the brain through the BBB and back into the circulation.
- As much as 90 percent of circulating Aβ peptide in healthy individuals is bound to albumin; albumin accounts for about more than one-half of total protein found in both blood and cerebrospinal fluid (CSF).
- Albumin undergoes glycation in normal aging, which attenuates its physiologic Aβ binding capacity; as a consequence, levels of toxic Aβ peptides may increase in the plasma and CSF.
- Glycation additionally attenuates the important antioxidant and antiinflammatory functions of albumin.10
- Both brain and plasma levels of glycated albumin have been found to be significantly higher in persons with AD than in age-matched controls.11
Plasmapheresis effects the removal of the patient’s “old” and presumptively less functional glycated albumin, along with thousands of other plasma elements. It is replaced with five percent human albumin sourced from plasma collected from relatively young healthy adult donors. Preliminary research suggests that this purified replacement albumin retains its Aβ binding capacity but contains no quantifiable levels of Aβ. The process of albumin replacement and plasmapheresis (plasma exchange) is repeated multiple times, with the goal of reducing brain amyloid burden through exploitation of the dynamic equilibrium between brain, CSF and plasma Aβ. Each removal of old glycated, Aβ-saturated albumin from the circulation and replacement with fresh albumin acts to create a concentration gradient that promotes mobilization and transit of neurotoxic Aβ already resident in the brain and CSF across the BBB, and into the circulation until a new brain-CSF-plasma Aβ equilibrium is established.
Pilot and proof-of-principle studies. Over the last decade marked by much excitement (not to mention investor interest) surrounding large-scale clinical investigations of several highly touted monoclonal antibodies targeted against Aβ, Grifols has quietly advanced its plasma exchange/plasmapheresis research platform. In 2008, Grifols collaborator Dr. Mercè Boada and colleagues at the University Hospital Vall d’Hebron in Barcelona presented results of an exploratory study of plasma exchange in seven AD patients. At 12 months following a maximum of six treatment cycles over a 21-day period, neurocognitive retesting at 12 months revealed a strong trend toward stabilization of cognitive function. In a surprising additional finding, six of seven patients showed a significant increase in cerebral perfusion in the frontal and temporal areas of the brain.12
Shortly thereafter, Boada published interim results from a randomized, sham-controlled trial of 23 patients with AD, who underwent a total of 18 plasma exchange procedures in just over 20 weeks. Measured using two standard scales (ADAS-Cog and MMSE), again a trend toward cognitive function differences favoring the plasma exchange group persisted throughout treatment and continued to one year follow-up.13
The AMBAR trial. Having demonstrated that this procedure is feasible, safe and shows promise of therapeutic benefit, Grifols initiated its pivotal Alzheimer Management by Albumin Replacement (AMBAR) study in early 2012. This ambitious clinical trial (Figure 1) reflects several significant refinements. Following an intensive treatment phase with six weekly “full volume” plasma exchanges, patients will be switched to monthly maintenance “low-volume” (650 to 800 mL) plasma exchanges (LVPEs). This faster “low volume” procedure was developed in collaboration with Fenwal, which has designed and provided a prototype plasmapheresis device specifically intended for this purpose.
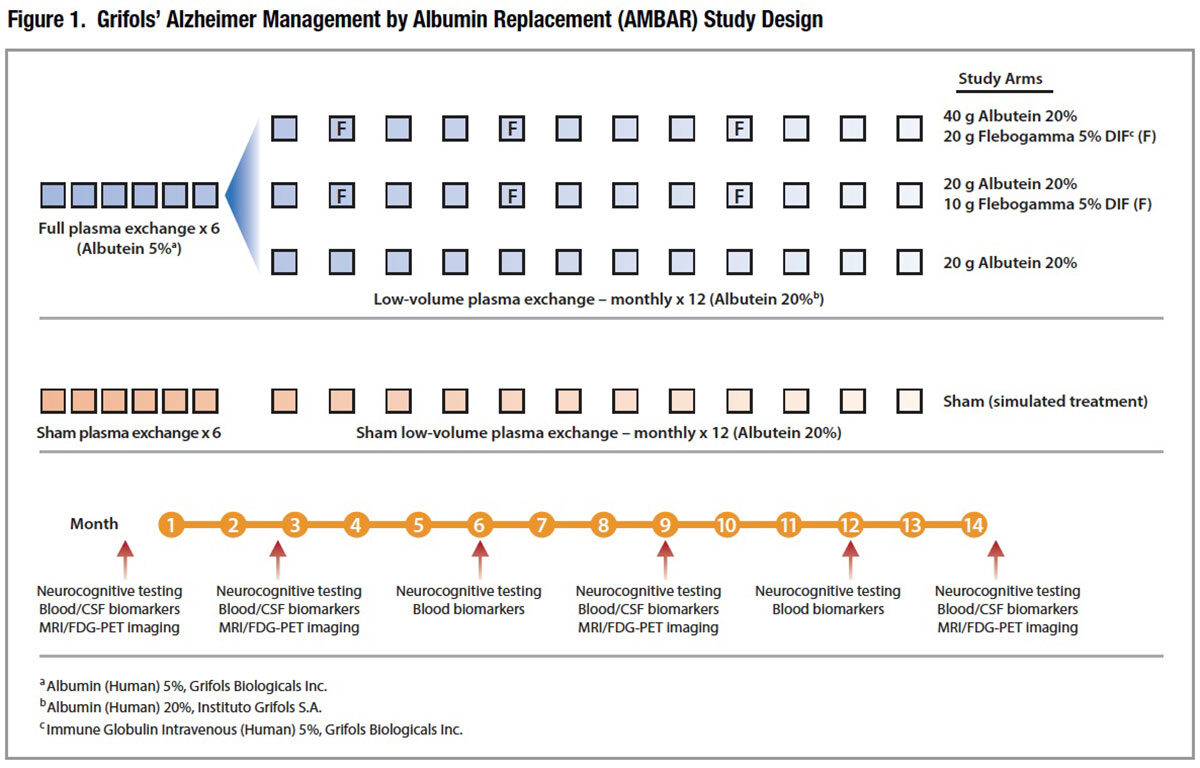
A total of four study arms, including a sham treatment arm, will allow investigators to evaluate alternative 20 percent albumin replacement strategies in the LVPE treatment phase, as well as supplemental immunotherapy with intravenous immune globulin (IVIG). Scheduled to enroll 350 subjects through the end of 2016, the AMBAR study is currently enrolling patientswith probable mild to moderate AD in centers in Spain and the U.S.
When It Is Better to Partake Than to Understand
Aging is often not kind. But modern medicine has found innovative ways to replace many failing body systems: healthy transplanted organs and tissues, artificial hips and knees, insulin to replace non-functioning pancreatic islet cells, hemodialysis when kidneys can no longer filter waste products from the blood.
AD presents an unusually daunting challenge: At best, we still have only a foggy notion of its underlying pathophysiology, a reality brought into sharper focus by the spectacular failures of three humanized monoclonal antibodies along with a host of other narrowly targeted “magic bullet” treatments. But it isn’t always necessary to understand, if observation teaches us how to treat. Human vaccine therapy traces its origins to the 18th century and Dr. Edward Jenner’s chance observation that milk maids infected with cowpox were resistant to England’s deadly smallpox epidemic; only centuries later would science explain how his crude cowpox vaccine actually works. In 1980, Swiss physicians were surprised to discover that human IVIG infusions in a boy with congenital agammaglobulinemia who happened to be also affected with immune thrombocytopenic purpura (ITP) dramatically increased his platelet counts; today, six IVIG products include an ITP indication. And, of course, plasma exchange is a standard first-line therapy for a number of serious autoimmune neurological disorders, even as its exact mechanisms of action remain a mystery.
In May 2014, Dr. Tony Wyss-Coray and colleagues at Stanford reported that exposure of old mice to plasma from young mice reversed “pre-existing effects of brain aging at the molecular, structural, functional and cognitive level.”14 Simple behavioral experiments confirmed that anatomical and biochemical changes in the hippocampus were accompanied by improved spatial learning and memory in the aged animals. “It was as if these old brains were recharged by young blood,” Wyss-Coray said.
Imagine that. Well, Grifols and its research collaborators already have, applying their own twist on “young-for-old” plasma protein replacement to focus primarily on human albumin, the predominant circulating blood protein also known to be critical for toxic Aβ clearance. By the end of 2015, the company plans to present interim results from its AMBAR trial.
Should Grifols’ novel plasma exchange-based approach prove to importantly slow AD progression, it could take many more years of study to elucidate exactly how it works. But we can be certain of one thing: Amid the rush to start treatment, neither affected patients nor their physicians or their loved ones will much care.
References
- National Center for Health Statistics. Health, United States, 2013. Hyattsville, MD. 2014.
- Alzheimer’s Association. 2014 Alzheimer’s Disease Facts and Figures. Accessed 11/7/2014 at www.alz.org/downloads/Facts_Figures_2014.pdf.
- Koyama A, Okereke OI, Yang T, et al. Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 2012;69:824-31.
- Sundelöf J, Giedraitis V, Irizarry MC, et al. Plasma beta amyloid and the risk of Alzheimer disease and dementia in elderly men: a prospective, population-based cohort study. Arch Neurol 2008 Feb;65(2):256-63.
- Lui JK, Laws SM, Li QX, et al. Plasma amyloid-beta as a biomarker in Alzheimer’s disease: the AIBL study of aging. J Alzheimers Dis 2010;20(4):1233-42.
- Pérez-Grijalba V, Pesini P, Monleón I, et al. Several direct and calculated biomarkers from the amyloid-β pool in blood are associated with an increased likelihood of suffering from mild cognitive impairment. J Alzheim Dis 2013;36:211-19.
- Games D, Adams D, Alessandrini R, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717f beta-amyloid precursor protein. Nature 1995;373:523-7.
- Town T. Alternative Aβ immunotherapy approaches for Alzheimer’s disease. CNS Neurol Disord Drug Targets 2009 Apr;8:92):114-27.
- Howcroft TK, Campisi J, Louis GB, et al. The role of inflammation in age-related disease. Aging (Albany NY) 2013 Jan;5(1):84-93.
- Roche M, Rondeau P, Singh, NR, et al. The antioxidant properties of serum albumin. FEBS Letters 2008;582(13):1783-7.
- Ramos-Fernández E, Tajes M, Palomer E, et al. Posttranslational nitro-glycative modifications of albumin in Alzheimer’s disease: implications in cytotoxicity and amyloid-β peptide aggregation. J Alzheimers Dis 2014;40(3):643-57.
- Boada M, Munoz J, Tárraga L, et al. P4-355: An exploratory study of the potential utility of plasma exchange with 5 percent human albumin Grifols in the treatment of Alzheimer’s disease (AD). Alzheimers Dement 2008 Jul;4(4 Suppl):T777.
- Boada M, Ortiz P, Anaya F, et al. Amyloid-targeted therapeutics in Alzheimer’s disease: use of human albumin in plasma exchange as a novel approach for Aβ mobilization. Drug News Perspect 2009 July-Aug;22(6):325-39.
- Wyss-Coray T, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 2014 Jun;20(6):659-63.