Superbug Apocalypse: A Post-Antibiotic Era?
- By Ronale Tucker Rhodes, MS
IT WAS CONSIDERED nothing short of miraculous when, in 1940, Oxford University scientists Howard Florey and Ernst Chain figured out how to isolate the antibacterial element penicillin, discovered in 1928 by Alexander Fleming in a discarded petri dish contaminated by mold. More than a decade after the “wonder drug’s” discovery, penicillin was at last able to be used to cure infections caused by deadly bacteria. Since its first use in 1942 during World War II, it is estimated penicillin has saved at least 200 million lives.1
But now, scientists are questioning just how miraculous penicillin, as well as the other classes of antibiotics, really are. Since bacteria’s discovery, strains have continued to evolve, making them more and more resistant to conventional antibiotics. According to the U.S. Centers for Disease Control and Prevention (CDC), each year, these drug-resistant bacteria infect more than two million people in the U.S. and kill at least 23,000.2 Indeed, only one antibiotic, colistin, which is used only when all other antibiotics fail because of its harsh side effects, has been known to work against particularly dangerous types of superbugs, including a family of bacteria known as carbapenem-resistant Enterobacteriaceae (CRE), which health officials have dubbed “nightmare bacteria.”3
Until now. In May 2016, the antibiotic-resistant strain was found in the urine of a 49-year-old Pennsylvania woman who Defense Department researchers determined carried a strain of Escherichia coli (E. coli) resistant to colistin. Most recently, in September, a 70-year-old woman from Nevada died of an infection found to be immune to all 26 antibiotics available in the U.S.4
Does this spell the demise of antibiotics? Or, will better ones or even something different replace them?
History of Antibiotics
The first patient to be treated with penicillin was a policeman who contracted a severe staphylococcal infection. It was 1941, and Florey and Chain were conducting the drug’s first clinical trial. After showing remarkable improvement, the policeman died, not because the penicillin didn’t work, but because supplies of it ran out after just five days. Because of its initial potential in the trial, production of penicillin expanded during World War II, helping to save thousands of soldiers’ lives. An advertisement in Life magazine in 1944, when the drug became available to the public, read, “Thanks to penicillin … he will come home!”5
Penicillin, it was found, was even more effective than previously discovered antimicrobial agents that reduced infection rates of diseases that once caused great pandemics such as the Black Death that killed 30 percent to 60 percent of Europeans in the 14th century and the 1918 Great Influenza pandemic that killed more than 50 million people around the world. Immediately, widespread use of penicillin ensued with the promise of preventing deaths caused by staphylococcus and streptococcus, and others that caused diphtheria, pneumonia and meningitis.6
But with its widespread use, resistance to penicillin quickly developed. In response, scientists introduced a new family of penicillin-type drugs, including one called methicillin in 1960. Discouragingly, a strain of staphylococcus resistant to methicillin, known as methicillin-resistant Staphylococcus aureus, or MRSA, developed within one year.6
The race to produce antibiotics, thus, began. Today, there are well over 100 antibiotics, the majority of which come from seven types of drugs, with 26 available in the U.S.7 But their explosive use in both humans and livestock has brought with it great consequence. According to award-winning journalist Maryn McKenna in her book Superbug: The Fatal Menace of MRSA: “It became evident that broad use of antibiotics not only caused drug-resistant infections; it also made people who had no symptoms of infection into silent carriers of drug-resistant strains.”6
Antimicrobial Resistance (AMR): Survival of the Fittest
When Fleming, Florey and Chain received the Nobel Prize in Medicine or Physiology, Fleming warned of antimicrobial resistance: “It is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them, and the same thing has occasionally happened in the body.”4 Indeed, Fleming’s warning proved true. Antibiotics are effective only when they are used as needed and as directed. Too much antibiotic use results in more resistant mutants of the bacteria. When the full course of antibiotics is cut short, the resistant strains multiply and spread.
How does bacterial resistance occur? It’s Darwin’s idea of survival of the fittest.
Resistance to antibiotics can occur in one of three ways: epigenetic adaptation, genetic adaptation and genetic acquisition, the latter of which is the major culprit of AMR. Epigenetic adaptation occurs when bacteria consistently encounter subinhibitory levels of an antibiotic, causing temporary resistance, but no permanent genetic changes that can be inherited by subsequent generations of bacteria. Genetic mutations, on the other hand, are permanent changes in genetic code that can occur due to a single mutation or multiple mutations. Resistance to some antibiotics can occur because of a single mutation, while other antibiotics require bacteria to develop multiple mutations.
Genetic acquisition occurs when bacteria acquire large chunks of foreign DNA that contain many genes. They acquire these through one or all of five techniques:
- plasmids, mobile pieces of DNA that bacteria can easily trade among themselves or acquire from the environment;
- transposons, sections of DNA that can jump from one place in the genetic code to another, or even to the genetic code of another organism;
- viruses, which can infect bacteria by copying and pasting genetic code into the genomes of the bacteria they infect;
- conjugation, which occurs when two bacteria directly adjacent to each other share DNA; and
- naked DNA, which bacteria find in the environment and internalize.8
The unique ability of genetic material to transfer DNA through plasmids is a great threat. While most genetic material is transmitted only from parent to offspring, plasmids can be transferred horizontally, from neighbor to neighbor. In fact, horizontal gene transfer in clinical settings is by far the most common mechanism through which bacteria become drug-resistant. “Resistance works differently in the bacterium,” explains Stuart Levy, director of the Center for Adaptation Genetics and Drug Resistance at Tufts University School of Medicine. “Their resistance can be transferred. It’s a little frightening to realize that you’re running after something that can transfer its football to somebody else right away.”5
The Dangerous Superbugs
In the past, some of the most dangerous superbugs have been confined to healthcare settings where people who are sick or in a weakened state are more susceptible to picking up infections. But superbug infections aren’t limited to hospitals.
In March 2015, the White House released a comprehensive plan outlining steps to combat drug-resistant bacteria. The plan identified 15 of the most dangerous superbugs, three of which pose an urgent threat, and the remaining 12 posing a serious threat (Table 1). On the urgent list are Clostridium difficile (C. diff), CRE and Neisseria gonorrhoeae (N. gonorrhoeae).9
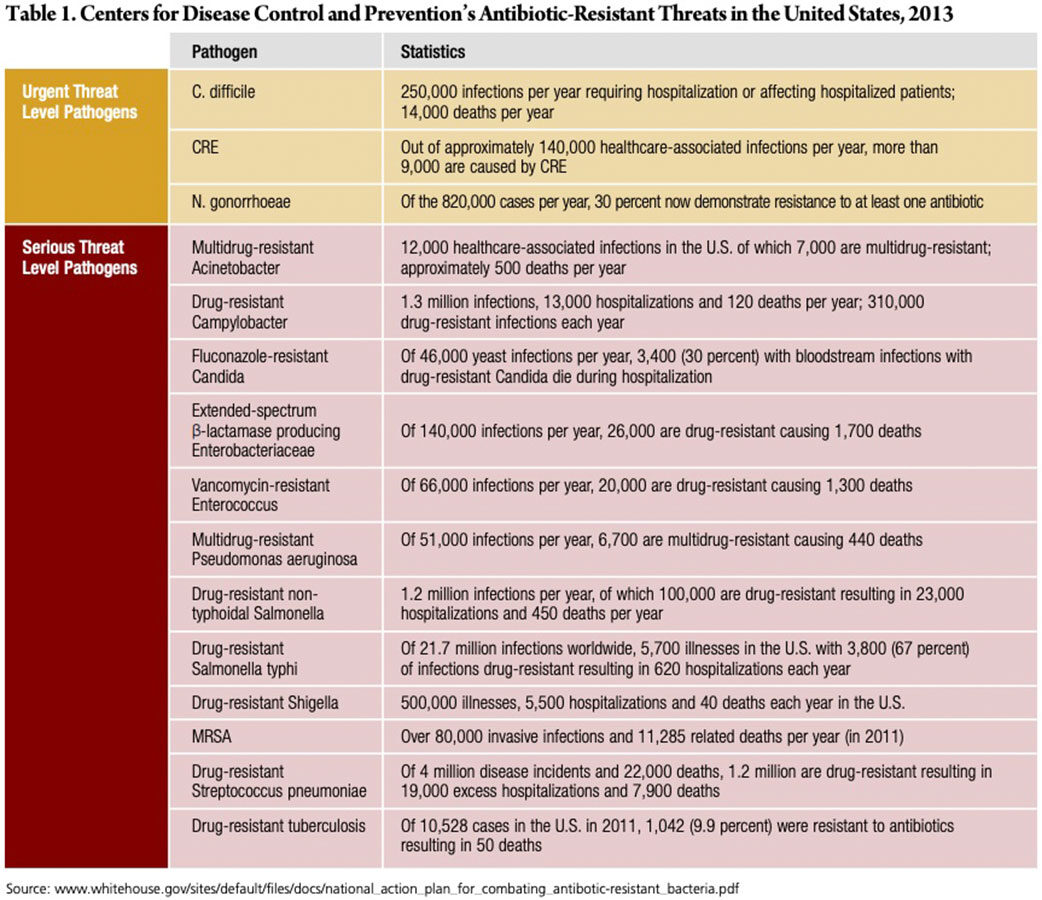
C. diff is a bacteria that lives in the intestines, causing life-threatening diarrhea, and is mostly contracted by people receiving medical care. Previously, doctors used antibiotics called fluoroquinolones to treat C. diff, but they don’t always work. From 2000 to 2007, deaths spiked 400 percent when a new drug-resistant strain of C. diff appeared.10
CRE is a family of bacteria normally found in the gut (e.g., E. coli), and mostly occurs in people who are in the hospital or in a medical care facility such as a nursing home. CRE is resistant to all antibiotics, resulting in death in up to 50 percent of patients who contract it, 10 including the 70-year-old Nevada woman who died in September. This woman was infected with a bacteria called Klebsiella pneumoniae, which belongs to the CRE class of drug-resistant bugs. She had been hospitalized in India just two months previously, one of several hospitalizations due to complications from a thighbone fracture. Upon returning to the U.S., she was admitted to a Reno hospital, and after doctors tried switching antibiotics accordingly, she died of septic shock.11
CRE is particularly troubling because a recent study found that the superbug may be spreading more widely than previously thought. A Harvard-MIT research team examined genetic sequences from approximately 250 samples of patients who had CRE in four hospitals in Boston and Irvine, Calif., over a 16- month period. They found little evidence of direct transmission between patients who became sick, leaving them to believe transmission may be occurring without causing symptoms. This means people colonized with these germs may spread them without ever becoming sick. According to Alex Kallen, MD, a medical officer in the CDC’s Division of Healthcare Quality Promotion, “the most common source of transmission with CRE is asymptomatic.”12
N. gonorrhoeae is a sexually transmitted disease that commonly spreads during oral, anal or vaginal contact. Pregnant women can pass the infection to their babies during childbirth. And, people can spread the bacteria without knowing it. While it used to be treated with antibiotics, the bacteria are becoming more resistant to current drugs.10
Antibacterial threats on the serious list include multidrug-resistant Acinetobacter, drug-resistant Campylobacter, fluconazole-resistant Candida, extended spectrum β-lactamase producing Enterobacteriaceae, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas aeruginosa, drug-resistant non-typhoidal Salmonella, drug-resistant Salmonella typhi, drug-resistant Shigella, MRSA, drug-resistant Streptococcus pneumoniae and drug-resistant tuberculosis.9
Who’s to Blame?
Regrettably, we’ve overused these “miracle drugs” to the point that they’ve lost their potency. A CDC study conducted in 2013 found that U.S. doctors are prescribing enough antibiotics to give them to four out of five Americans every year. Specifically, it found that doctors and other healthcare providers prescribed 258 million courses of antibiotics in 2010 for a population just shy of 309 million, which translates to 833 antibiotic prescriptions for every 1,000 people, on average. The study also found that the most frequently prescribed antibiotic was azithromycin, which is commonly used for bronchitis symptoms. The problem with this is bronchitis is usually caused by a virus, and antibiotics like azithromycin don’t work against viruses.13
In 2015, the most in-depth study yet to examine the use and misuse of antibiotics found nearly one-third of antibiotics prescribed in doctors’ offices, emergency rooms and hospital-based clinics in the U.S. are not needed. This means approximately 47 million unnecessary prescriptions are given for conditions that don’t respond to antibiotics. The study analyzed data collected from two major CDC surveys from 2010 to 2011. It found that 13 percent of all outpatient visits in the U.S. (approximately 154 million annually) result in an antibiotic prescription; more than four in 10 (44 percent) are written to treat patients with acute respiratory conditions; and half of these prescriptions are unnecessary because they are for viral illnesses. What’s more, an accompanying editorial published in the same journal issue as the study noted the numbers are likely an undercount because they don’t include the times antibiotics are given when patients talk to doctors over the telephone or when they seek medical care at urgent care clinics, retail pharmacies and dental offices.14
But, it’s not just overuse. Antibiotic resistance is facilitated by a combination of two other factors: environments and hosts.
Hospitals are the perfect environment for bacteria to develop, acquire and maintain high-level antibiotic resistance because there are a lot of infected people and contaminated surfaces, a high number of patients who are potential hosts and frequent and sustained use of antibiotics. Not only are bacteria spread among patients, but bacterial infections are associated with procedures like surgery and devices used in medical procedures. Despite the sterility of hospitals, hospital-acquired infections are one of the leading causes of morbidity among patients in the U.S.8
Antibiotic resistance is in large part contributed to by industrial animal farming, which consumes more antibiotics than are used in human medicine. Indeed, 80 percent of antibiotics in the U.S. are used on livestock, with North Carolina farm animals receiving more than all Americans combined.15 In these operations, antibiotics are used prophylactically to prevent disease outbreaks. But, the facilities mimic unsanitary and overcrowded hospitals often found in developing countries, which drives the evolution of antibiotic resistance in bacteria. What’s more, animal waste contains significant levels of unmetabolized antibiotics, which encourages the transfer of antibiotic resistance genes among different species of bacteria. And, it doesn’t stop there. These facilities cause the spread of antibiotic resistant bacteria to neighboring wildlife and in rivers, lakes and other waterways.8
Looking at Solutions
The frightening rise in antibiotic resistance has been growing for decades. So, why isn’t more being done to counteract the problem before there are no other alternatives? Pharmaceutical companies say they are at least partially to blame for AMR by neglecting to develop new and more sophisticated antibiotics that could keep up with bacterial resistance. The reason: There’s not much money to be made with antibiotics. There’s a lot more money to be made in cancer, diabetes and hypertension drugs, for example, that have small margins but generate profits over time because patients use them for life.16 Global sales of antibiotics areroughly $4.7 billion a year, about as much as a singletop-selling cancer drug.17 Since the correct way to use an antibiotic is only briefly, from an economic standpoint, the developer is not getting the return on its investment. It costs between $600 million and $1 billion to bring a drug to market, so it makes sense for drug developers to turn to other drugs to bring revenue back to their shareholders.18
Aaron Kesselheim, director of the Program on Regulation, Therapeutics and Law at Brigham and Women’s Hospital, suggests changing the current model for reimbursement. Currently, drugs are reimbursed on a per-prescription basis, which encourages the overprescription of antibiotics. The goal of a new model, he says, should be to get a return on investment, as well as ensuring the conservation of antibiotics that are needed for future generations — not overusing them during the initial period of market exclusivity, when it makes the most financial sense to companies that develop the drugs. That would mean “linking the patent life to actual prudent use of the antibiotics and appropriate use of the antibiotics,” says Kesselheim. “Right now what happens is when a new antibiotic hits the market, the pharmaceutical companies are interested in promoting the product as much as possible, and getting as much prescription of it as possible for that limited period of time that they have exclusive rights over it. And that’s not consistent with public health goals.”18
In years past, the U.S. Food and Drug Administration (FDA) and CDC launched antibiotic resistance campaigns aimed at healthcare professionals and the public to discourage overprescribing and overuse. And, a nationwide ad campaign developed by FDA’s Center for Drug Evaluation and Research emphasizes to healthcare professionals the prudent use of antibiotics and offers them an educational brochure to distribute to patients.19 But, clearly, as evidenced by CDC studies, overprescribing and overuse persists.
Heightened consumer awareness of the superbug threat, however, appears to be altering consumer behavior somewhat. In 2014 and 2015, sales of antibiotic-free meats jumped 20 percent.15 And, government is stepping in to curb the use of antibiotics in livestock, too. In December 2013, FDA put in place a major new policy to phase out the indiscriminate use of antibiotics in cows, pigs and chickens raised for meat. Phased in over three years, the policy made it illegal for farmers and ranchers to use antibiotics to make animals grow bigger. Additionally, FDA required that licensed veterinarians supervisethe use of antibiotics, requiring farmers and ranchers to obtain prescriptions to use the drugs for their animals.20 Most recently, regulations are being enacted to shift to antibiotic-free meats. In October 2015, California passed a strict law limiting the use of antibiotics in agriculture.15
A report commissioned by United Kingdom Prime Minister David Cameron and the Wellcome Trust in May 2016, which outlines how to tackle the spread of AMR and how to pay for it, is attracting the attention of world leaders. The report calls for a “two-tiered approach: Lower the use of available antimicrobials, and increase the supply by stimulating the development of new ones.” According to the report, reducing use could be achieved by improving hygiene, faster and better diagnostics and new vaccines. In addition, a worldwide campaign to educate consumers about the dangers of resistance could keep patients from demanding the drugs and doctors from prescribing them. The report also calls for not using antibiotics in agriculture at all. And, it offers concrete suggestions for how to boost the development of drugs effective against resistant infections: “A Global Innovation Fund endowed with up to $2 billion is needed to fund early-stage research; a bonus of $1 billion for a company that develops a new drug that is effective against resistant infections could also help. The money could be raised as a levy from these companies through a ‘pay-or-play’ strategy, in which companies can either pay up or invest it in research and development to fight AMR.”17
Still, 80 years after the discovery of penicillin, it is still not really known how to develop a good antibiotic. And, the responsibility is falling increasingly to academic researchers. Harvard’s Program on Antibiotic Resistance organized in 2009 has seven independent labs that study antibiotic-resistant S. aureus. Its goal is not necessarily to develop new drugs, but to develop innovative approaches to finding them. “We explore new drug targets that are higher risk than those a company would work on,” explains Suzanne Walker, professor of microbiology and immunology. “It’s hard to beat a company at developing a compound, and there’s no reason to do that. But I think it’s up to academics to lay the groundwork.”5
But not all academic researchers are going down that path. At Stanford University’s Chemistry, Engineering and Medicine for Human Health Institute, a group of undergraduate students is working on “novel antibiotics from scratch that might one day stand up to superbugs.” They are focusing on two bacteria with high mortality rates that are resistant to nearly all antibiotics: Pseudomonas aeruginosa and Acinetobacter baumannii.16
There also are some drug developers that are making headway. France-based Eligo Bioscience is researching a potential CRISPR-based solution to the AMR problem; Iterum Therapeutics is launching an antibiotic licensed by another manufacturer called Dalvance that is used to treat adults with skin infections; and in September 2016, AstraZeneca, GlaxoSmithKline, Johnson & Johnson, Merck & Co., Novartis AG, Pfizer and Sanofi SA were among the signatory companies to an “Industry Roadmap for Progress on Combating Antimicrobial Resistance.”16
A Five-Year Plan
The threat to the usefulness of antibiotics can’t be overstated. By 2050, it is estimated that AMR-related deaths could total 10 million annually (cancer kills about 8.2 million people worldwide annually).16 And, the global economic tally from antibiotic-resistant bacteria could be as high as $100 trillion.15 Politicians and the medical community agree that to counteract the threat of superbugs, abuse in both medicine and agriculture must be restrained, and new drug development must be stimulated.
Last year, the Obama administration announced a five-year plan to combat superbugs, asking Congress to double funding to $1.2 billion. In the “National Action Plan for Combating Antibiotic-Resistant Bacteria,” the administration set a target of reducing inappropriate antibiotic use in outpatient settings by half by 2020, resulting in approximately 23 million fewer antibiotics prescribed annually. According to the report, the plan’s goals are to “slow the emergence of resistant bacteria and prevent the spread of resistant infections; strengthen national One-Health surveillance efforts to combat resistance; advance development and use of rapid and innovative diagnostic tests for identification and characterization of resistant bacteria; accelerate basic and applied research and development for new antibiotics, other therapeutics and vaccines; and improve international collaboration and capacities for antibiotic-resistance prevention, surveillance, control and antibiotic research and development.”9
References
- New World Encyclopedia. Alexander Fleming. Accessed at www.newworldencyclopedia.org/entry/Alexander_Fleming.
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance. Accessed at www.cdc.gov/drugresistance/index.html.
- Gano R. E-Coli Superbug In US — Totally Resistant To Antibiotics — The End of The Road. Prophezine, May 26, 2016. Accessed at raygano.com/breaking-news-e-coli-superbug-in-us-totally-resistant-to-antibiotics-the-end-of-the-road.
- Kirkey S. Superbug Immune to Virtually Every Known Antibiotic Kills U.S. Woman. National Post, Jan. 13, 2017. Accessed at news.nationalpost.com/news/0114-na-superbug.
- Xue K. Superbug: An Epidemic Begins. Harvard Magazine, May-June 2014. Accessed at harvardmagazine.com/2014/05/superbug.
- Meakin J. Superbugs! The End of the Antibiotic Era. Tomorrow’s World, January-February 2016. Accessed at www.tomorrowsworld.org/magazines/2016/january-february/superbugs-the-end-of-the-antibiotic-era.
- eMedicineHealth. Types of Antibiotics. Accessed at www.emedicinehealth.com/antibiotics/page2_em.htm.
- Science of Acne. How Do Bacteria Become Resistant to Antibiotics? Accessed at thescienceofacne.com/how-dobacteria-become-resistant-to-antibiotics.
- The White House. National Action Plan for Combating Antibiotic-Resistant Bacteria, March 2015. Accessed at www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_ bacteria.pdf.
- Miller K. Superbugs: What They Are and How You Get Them. WebMD, April 17, 2015. Accessed at www.webmd.com/a-to-z-guides/news/20150417/superbugs-what-they-are#1.
- Mohny G. What You Need to Know About the Deadly ‘Superbug’ Infection Resistant to All FDA-Approved Antibiotics. Yahoo News, Jan. 16, 2017. Accessed at gma.yahoo.com/know-deadly-superbug-infection-resistant-fda-approved-antibiotics-233300910–abc-news-wellness.html.
- Scutti S. Drug-Resistant Superbug May Be More Widespread Than Previously Known. CNN, Jan. 17, 2017. Accessed at www.cnn.com/2017/01/16/health/cre-superbug-disease-study/index.html.
- Stobbe M. Study Shows Overuse of Antibiotics. USA Today, April 10, 2013. Accessed at www.usatoday.com/story/news/nation/2013/04/10/medication-antibiotic-overuse/2071899.
- Sun LH. 1 in 3 Antibiotics Prescribed in U.S. Are Unnecessary, Major Study Finds. The Washington Post, May 3, 2016. Accessed at www.washingtonpost.com/news/to-your-health/wp/2016/05/03/1-in-3-antibiotics-prescribed-inu-s-are-unnecessary-major-study-finds/?utm_term=.ebcc8225a488.
- Mansharamani V. Superbugs: The $100 Trillion Risk. Fortune, June 1, 2016. Accessed at fortune.com/2016/06/01/antibiotic-superbugs-bacteria-e-coli.
- Dittman D. Superbugs: How Antibiotic-Resistant Bugs Are Killing Mankind. Wall Street Daily, Oct. 19, 2016. Accessed at www.wallstreetdaily.com/2016/10/19/superbugs-antibiotic-resistant-bacteria.
- Kupferschmidt K. Long-Awaited Report Outlines How to Fight Antimicrobial Resistance — And How to Pay for It. Science, May 18, 2016. Accessed at www.sciencemag.org/news/2016/05/long-awaited-report-outlines-how-fight-antimicrobial-resistance-and-how-pay-it.
- Battling Drug-Resistant Superbugs: Can We Win? The Forum at Harvard School of Public Health, Feb. 5, 2014. Accessed at theforum.sph.harvard.edu/events/battling-drug-resistant-superbugs.
- U.S. Food and Drug Administration. Battle of the Bugs: Fighting Antibiotic Resistance. Accessed at www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm143568.htm.
- Tavernise S. F.D.A. Restricts Antibiotics Use for Livestock. The New York Times, Dec. 11, 2013. Accessed at www.nytimes.com/2013/12/12/health/fda-to-phase-out-use-of-some-antibiotics-in-animals-raised-for-meat.html.