The Perfect Storm for Patient-Focused Clinical Trials
Improving clinical trial enrollment numbers is a key challenge to advancing research, but a host of solutions suggests a new era of patient engagement and patient-focused clinical trials.
- By Tina Tockarshewsky
“If patient engagement were a drug, it would be the blockbuster drug of the century and malpractice not to use it.”1 Digital health IT strategy consultant Leonard Kish’s bold 2012 statement heralds the new era of patient engagement in the medical research and development process. Buzz phrases like “patient-focused,” “patient-centric” and “patient-driven” are being bantered about, and while all process stakeholders agree that, theoretically, this patient-facing approach is critical, what those phrases actually mean in practice is still being defined. There is no denying the positive winds of change due to high stakes and a perfect cultural storm fueled by a drug development process that has not been working well.
The common denominator and greatest catalyst for this change is the ultimate end-user: the patient. Patients themselves and patient organizations have always stressed a greater need for patient engagement; however, clinical trial design and development is an inherently data-driven process that often disenfranchises its own end-user. Yet, despite being a numbers-driven process, the numbers are not adding up: Clinical trials are faltering at an alarming rate and with staggering costs.
According to Pharmaceutical Research and Manufacturers of America (PHRMA), in 2013:2
- There were 6,199 industry-sponsored clinical trials in the U.S., with 1.1 million participants.
- The U.S. biopharmaceutical industry had nearly $10 billion of direct spending in the conduct of clinical trials at the site level. This does do not include resource investments for clinical trial-related activities occurring outside the individual trial sites.
- Direct and indirect clinical trial investments by industry and clinical trial vendors and contractors generated $25 billion in local community economic activity.
- All 50 states and the District of Columbia had trials, with five states having the highest number of active sites: California (3,111), Texas (2,799), Florida (2,571), New York (2,476) and Pennsylvania (1,972).
Clinical trials are a crucial part of the drug development process, but they are costly, with expenses increasing exponentially as the trial moves through each phase. PHRMA data also show that trial sites tend to be more concentrated in key states having major urban centers (and, by extension, more accessible to those markets). Costs per trial participant can average $36,500 across all phases for each phase, but Phase I through Phase III can have higher per-trial participant costs, ranging from $38,500 to $42,000 per person.2
Still, despite this enormous investment, producing market deliverables is difficult. FasterCures, a Milken Institute think-tank center focused on accelerating research and removing barriers to medical progress, cites the following statistics:3
- One in three Americans lives with a deadly or debilitating disease that has no cure or few treatment options.
- In 2014, only 41 new drugs were approved despite an annual investment of $100 billion in therapeutic research and development.
- Only one out of every 10,000 scientific discoveries makes it to market.
Developing a new medicine takes, on average, 10-plus years and costs $2.6 billion.4 After adding time for basic science research and regulatory approvals, this nearly two-decades-long, high-cost process now constitutes a high-risk event facing enormous odds of even crossing the finish line. Many of those odds are dictated by patient engagement:5
- 80 percent of total trials are delayed at least one month because of unfulfilled enrollment.
- 50 percent of clinical research sites enroll one or no patients in their studies.
- Each day a drug is delayed from market, sponsors lose up to $8 million.
Federal Programs Push Progress
Several U.S. government programs are addressing patient engagement levels in drug development, and these programs are setting into motion new directions taken by industry as well.
Patient-Centered Outcomes Research Institute (PCORI). The Affordable Care Act of 2010 established PCORI, an independent nonprofit, non-governmental organization whose mission is to help “people make informed healthcare decisions, and improve healthcare delivery and outcomes, by producing and promoting high-integrity, evidence-based information that comes from research guided by patients, caregivers, and the broader healthcare community.” PCORI funds comparative clinical effectiveness research (CER), as well as supports methodology improvements for CER studies. Using an approach called Patient-Centered Outcomes Research (PCOR), the studies supported by PCORI address the questions and concerns most significant to patients and do so by involving all stakeholders — patients, caregivers, clinicians and other relevant healthcare parties — as well as researchers.6
PCORI has invested $250 million to develop PCORnet, a national patient-centered clinical research network, which aims to aggregate national data sourced from a range of healthcare settings (including local hospitals, doctors’ offices and community clinics) into a large, highly representative national network for conducting CER. Phase I of a two-phase PCORnet launch process started in 2014 to include Clinical Data Research Networks and Patient-Powered Research Networks, as well as a Coordinating Center led by Harvard Pilgrim Health Care Institute and Duke Clinical Research Institute. Phase II commences late 2015 with the inclusion of rare disease networks, as well as networks and communities with common conditions and/or shared attributes.7
Patient-Focused Drug Development (PFDD). First enacted in 1992, the Prescription Drug User Fee Act (PDUFA) aimed to streamline and expedite the U.S. Food and Drug Administration’s (FDA) new medicine approval process. The fifth authorization of PDUFA in 2012 mandated the framework for a new FDA initiative, called PFDD, intended to include patients in earlier stages of product development. The legislation called for FDA to enhance patient input in four drug development areas: 1) the benefit-risk framework, 2) patient-reported outcome endpoints (PROs) and other assessment tools used, 3) divisions review and 4) patient involvement in advisory committees, endpoint development and risk communications. While, currently, PFDD is limited to patient insights via 20 disease-specific meetings (the 20 were identified as those with greatest need through a public comment period to shorten a longer FDA-driven list), the patient-centered fashion in which the FDA initiative is designed is seen by many as influencing the pharmaceutical industry to evolve its own patient-centered drug development approaches.8
The Research Continuum
The PCORI and PFDD initiatives contribute to the growing trend to incorporate patients at each and every juncture, from lab bench to bedside. When it comes to involving patients and patient advocacy groups on the front end of clinical trial design, John Barnes, executive director of the Coalition for Clinical Trial Awareness, urges “that’s where the rubber hits the road for including patients, the patient’s voice, and patient’s family.”
In 2012, the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH) was established to tackle transforming translation — the process of turning discoveries from the laboratory, clinic and community into actual clinical applications — so new drugs, diagnostics, medical devices and, ultimately, cures could reach patients faster. NCATS does not focus on specific diseases but rather on common denominators among diseases. At the core of all of its translational science programs is the patient.
Petra Kaufmann, MD, MSc, director of the division of clinical innovation at NCATS, says: “We see research as a cycle, not a linear process. The patient has to be in the center and actively engaged throughout the process.” NCATS programs take into consideration all ways in which the patient is engaged with the development of their care (Figure 1). “Observations from patients inform the process,” Dr. Kaufmann adds, noting that it is a “continuous learning system” in which the most critical stakeholder is the patient.
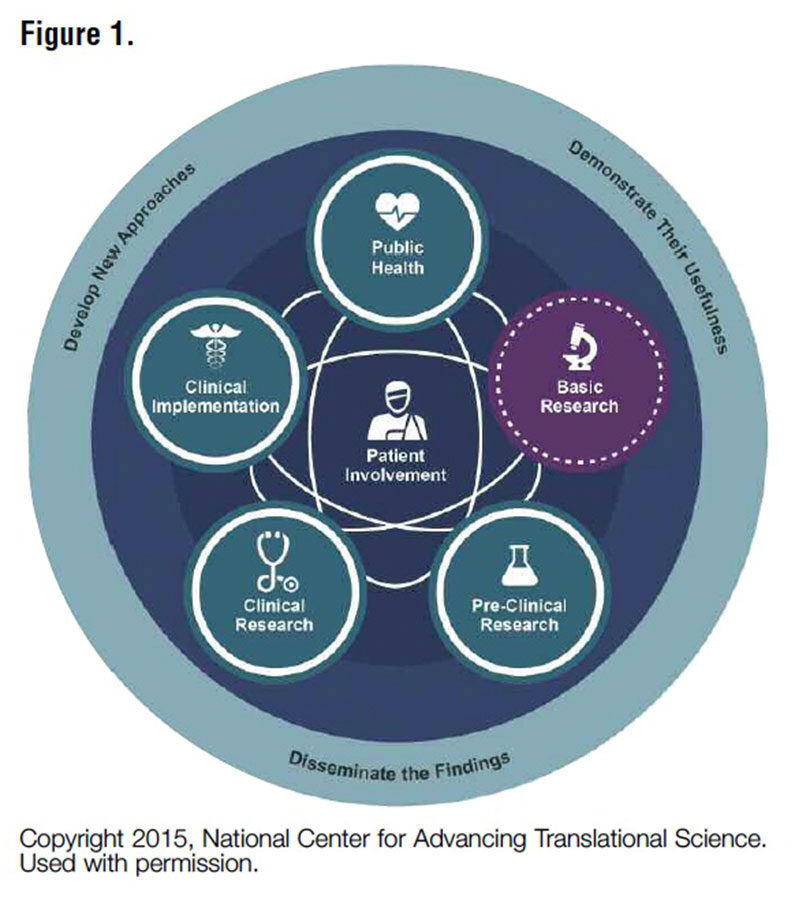
From Dr. Kaufmann’s perspective, a key challenge across the research continuum is that active engagement of patients is still a new thing. She observes that in many areas there is a lack of awareness by stakeholders of how to incorporate patient engagement and a lack of best practices in terms of the methods and processes this might involve. Specifically, she points out that:
- Patients may feel they do not have enough information to be active partners in research. “To bring more treatments to more patients, we need to engage patients as active partners in research, alongside scientists, industry and government. That requires giving patients and their family the tools and information they need to be empowered as an active participant,” Dr. Kaufmann observes.
- Raising awareness is needed among investigators to change patient engagement paradigms. Initiatives like NCATS’ Rare Disease Clinical Research Network require that at least one patient group is actively engaged in each of its consortia that work to find answers and treatments for rare diseases. The Clinical and Translational Science Awards (CTSA) program with its national consortium of medical research centers has been looking for innovation and best practices in connecting with all research stakeholders. As Dr. Kaufmann explains, “They all work on engaging communities and patients: We believe patient engagement is a transformative tool and a key part of our CTSA and Rare Disease Network programs.”
- New understandings of transparency issues are needed. As stakeholders find new ways to work together, some patient groups may have a learning curve in understanding the need for full disclosure of their network of relationships and funding sources to avoid any unintended potential conflicts of interest.
As another sign of positive change, Dr. Kaufmann points to her previous work with the NeuroNext research network at the National Institute of Neurological Disorders and Stroke. NeuroNext grant recipients are required to incorporate patient protocol monitoring groups and patient advocates during trial design and implementation. “It instills trust in the research process if advocates are on monitoring boards — it offers a two-way street to real change,” Dr. Kaufmann explains.
While she is encouraged by cultural shifts like pharma companies designating “chief patient officers,” Dr. Kaufmann hopes in the future there will be more sharing between public and private sectors to accelerate the development process.
Maximizing Patient Participation: More Education, Empowerment, Ease of Access
Despite good intentions and cultural changes, if the general public does not have a good foundation in understanding research, they will not get involved. And even those who do engage still face entry barriers that may exclude them.
Patient communities are trying to change this, both by educating their members and by joining together to call for national platforms to accelerate education. The Coalition for Clinical Trial Awareness (CCTA) is advocating for the creation of a federally sponsored public awareness campaign to explain the benefits of clinical trials. John Barnes, a member of CCTA’s management team, states the greatest impediment to truly developing patient-focused clinical trials is the lack of awareness of what clinical trials are. But another impediment to trial education and access, he notes, is that “doctors are hesitant to talk to patients; they feel they will lose their patients” if enrolled in a clinical trial (Figure 2).

Kim McCleary, managing director and leader for a new FasterCures program to advance the science of patient input and expand patient engagement in FDA’s assessment of benefits and risks for medical products, observes: “Patient engagement is still seen as a solution to a problem instead of a guiding philosophy, especially for clinical trial recruitment; by that time, it’s too late to address it when recruitment is not going well.”
McCleary points out that the timeline for the development process endpoints is extending: “Regulatory approval used to be considered the end of the process — it was the ‘Holy Grail.’ But now the timeline has shifted to include payers and providers and their impact on access to care. Reimbursement has not been focused on by patient organizations. Now, individuals are sharing more of the cost of healthcare, so they are more concerned about these issues.”
McCleary realizes stakeholders are hungry for best practices; however, she feels it is still too early, expressing that she sees stakeholders going through “a spirit of experimentation, a learning period and a shake-out period.” She notes the increasing interest in leveraging patient registries, with leading models like PCORI’s emphasis on patient-powered registries and the ability to link registries to ask a single research question across different communities as demonstrating the benefit these registries can provide. “PCORI is pushing the conversation at different levels, with patient organizations and with other players,” states McCleary. “They are showing leadership for foundational work involving patients, but is this something patients will value?” Ultimately, she acknowledges the perfect storm environment facilitating increased patient engagement: “There will be an inevitable societal and cultural shift of patient empowerment to shed a paternalistic system.”
Know Your Customer
While the public’s understanding of clinical trials is a major factor impacting enrollment, so too, in reverse, is investigators’ understanding of the public they seek to engage. Patient recruitment issues, both for volume and for finding appropriate candidates and avoiding “professional patients” with questionable motives and sketchy medical references, plague the process, frustrating investigators’ efforts to move forward. Even the best patient-focused trial design will not succeed if enrollment targets fail.
In a 2013 FDA Workshop on Peripheral Neuropathy Clinical Trials presentation, this author shared enrollment insights resulting from a patient community poll conducted by The Neuropathy Association:
- The key personal drivers for trial participation were access to leading researchers and healthcare providers (35 percent), receiving new therapies before they were publicly available (30 percent) and participating in research to help other patients (25 percent); remuneration motivated only 5 percent of those surveyed.
- Main reasons for not participating in clinical trials were lack of awareness of personally-applicable trials (27 percent), inability to travel (18 percent) and lack of access to general trial information (13 percent).
- The patient community likes being proactive partners in their care (i.e., using tools/resources like tracking mechanisms for charting pain and mapping progress) (Figures 3 and 4).
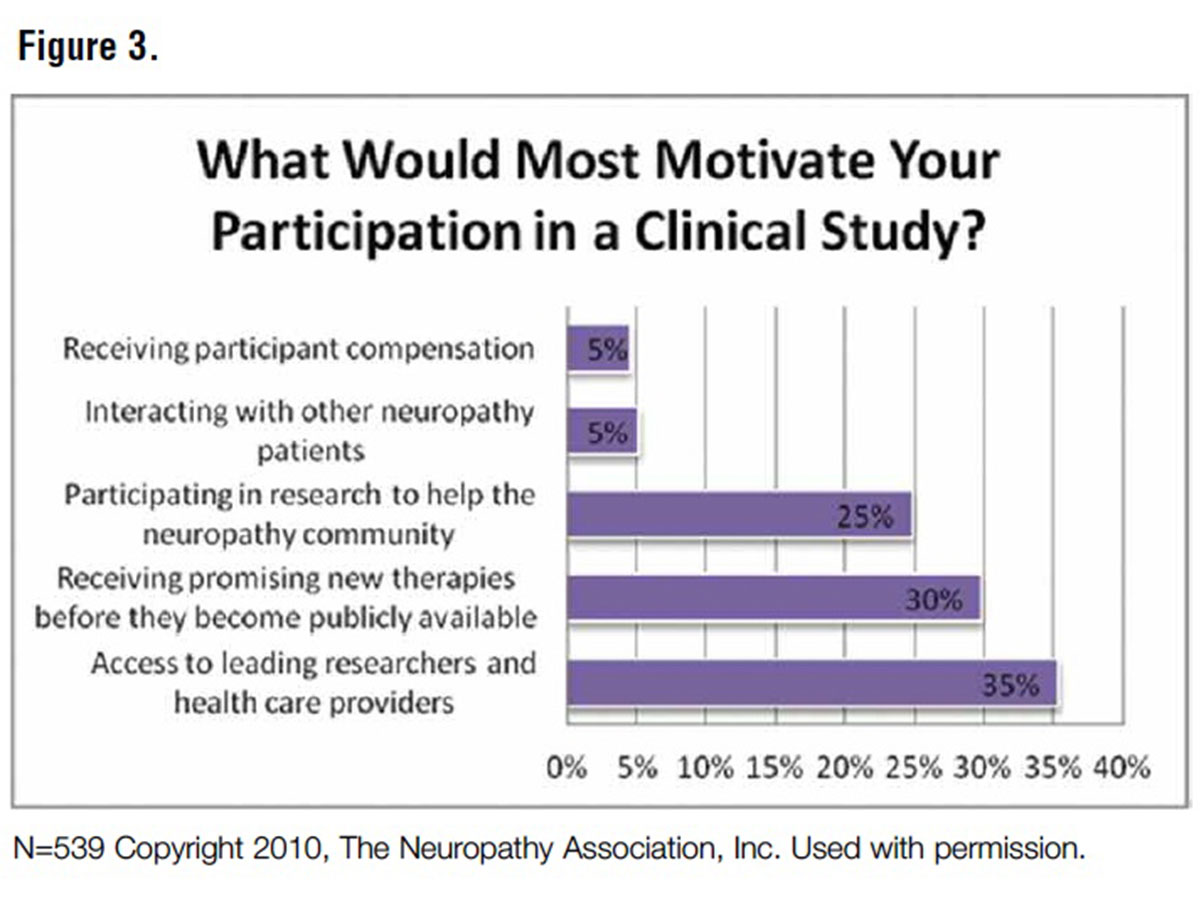
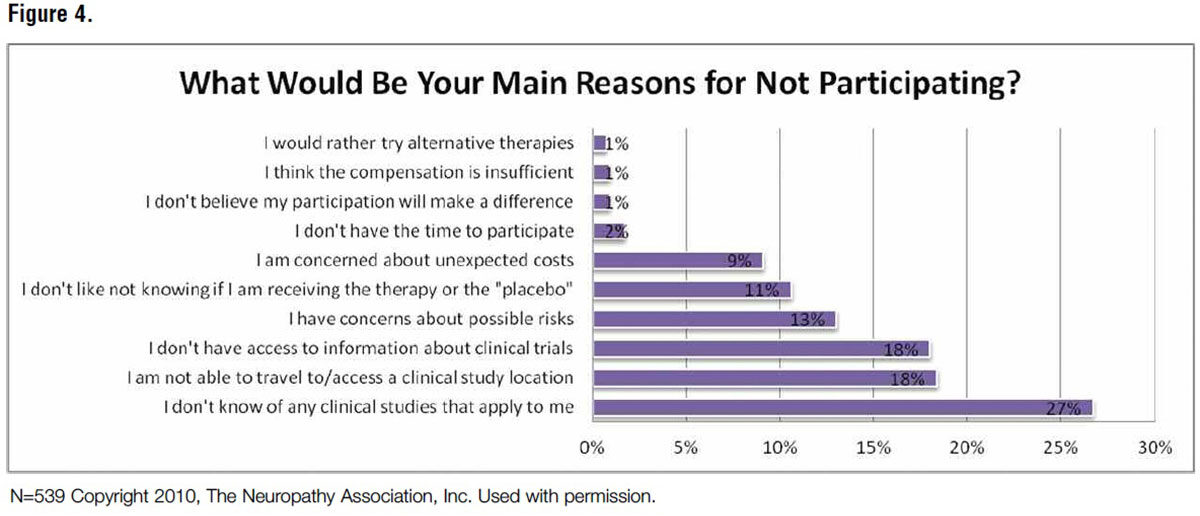
The poll and resulting presentation outlined neuropathy patients’ most challenging barriers to trial participation:
Fear. After enduring numerous challenges to get to a diagnosis and a treatment regimen with a certain level of symptom management, neuropathy patients’ greatest fear was having to stop or upset their therapeutic balance (even if imperfect). There was also the fear of the unknown — the risks of not tolerating a new therapy or getting worse during a trial.
Access and costs. Trial access had another meaning for these patients: Physical, financial and support impediments limited their trial access. Many did not have the physical stamina or mobility to travel to trial sites, often depending on others or challenging public transportation options. With many already on disability or struggling with job absences due to illness, asking them or their family and friends to sacrifice time away from work or time away from their family presented a huge hidden cost burden.
Awareness. Despite proactive outreach to their physicians — whom they viewed as stewards in encouraging trial participation — these patients were disappointed by the lack of engagement or support from their treating physicians, as well as their perception that physicians discounted the disease’s impact on their lives.
These points come from a specific disease community, yet contain common themes across illnesses. And the points raised show the value of soliciting patient input about the dynamics within a disease population.
Patient organizations stand ready to help with recruitment efforts to pinpoint targeted patient populations. They are vested in their communities, and they know how to find one another. Today, social media is an enormous aggregator: Patients want to help other patients, peers and those with analogous illnesses, overlapping disease states or shared comorbidities. Trial recruitment efforts have barely scratched the surface in exploring how patient social networks could be leveraged to extend outreach and patient engagement.9
Details Driving Data
For those actually designing clinical trials, how to involve patients in design is still a wide playing field open for consideration. Robert Dworkin, PhD, professor in the departments of anesthesiology and neurology and center for human experimental therapeutics at the University of Rochester Medical Center and co-director of the Analgesic, Anesthetic and Addiction Clinical Trial Translations, Innovations, Opportunities and Networks (ACTTION) public-private partnership with FDA, says of analgesic trials that one might consider parallel versus crossover, enriched enrollment and randomized withdrawal designs, as well as designs in which patients are offered choices of treatments. He says that there is great interest in options for “phenotyping patients in various ways — identifying specific subgroups of patients who might respond better or tolerate the treatment better than other patients.”
Patient organizations have an opportunity to help researchers and FDA refine this approach. They can provide researchers with what they know about differences in their own patient subpopulations and about varying levels of risk tolerance across their disease’s cycles and progressions — from both the patients’ and caregivers’ perspectives. Researchers can harness these insights to improve their design efforts by asking patients’ help with 1) generating hypotheses, 2) developing outcome measures and 3) assessing benefit-risk value propositions. Researchers trained to listen to the voice of the patient and to map patients’ symptom articulation and desired outcomes can combine this input with their methodological “know-how” to channel the information into rigorous clinical trial studies.9
Patient reported outcomes (PROs) are another area of intense focus for process improvements. And, as the process timeline extends out to payers, this is an area where payers could benefit from earlier involvement in a patient-focused drug development process. The biggest challenge for payers making drug coverage and formulary decisions is how to generalize study findings to patients who are different (but perhaps more prevalent) than those enrolled in pre-launch studies. Information from a patient-focused development process can aid payers, helping them better interpret product information, contextualize PRO data and generalize study data to address varied patient populations. Irrespective of the stakeholder, as with other areas, best practices and guidance for PROs are still a work in progress.9
Make Way for Disruptors and Innovators
Patient engagement is being bolstered by new technologies. Wearable devices, GPS and tracking technologies, video conferencing, mobile phone apps and other direct-to-consumer devices are being brought into the domain of research for data measurement and collection. Apple’s ResearchKit has already expressed its intent to bypass the clinical middleman by offering an open source software framework making app creation for medical studies easier for researchers and developers. The tidal wave of new applications and new uses for technology and data has only just begun, and the opportunities are immeasurable.
Incorporating new technologies and consumer devices to drive patient engagement is still, at best, at a point of experimentation, with best practices still a ways off — but the commitment by stakeholders to try new protocols is there. In 2011, Pfizer announced it was moving forward with a first-ever, fully at-home and completely virtual randomized clinical trial called REMOTE (Research on Electronic Monitoring of OAB Treatment Experience) for their overactive bladder drug Detrol LA (tolterodine tartrate). With a goal to recruit 600 patients from 10 U.S. states, all aspects of the trial were to be “virtual.” Recruitment and sign-up were done online, drugs would be mailed to patients’ homes, data would be collected via computer or smartphone, and blood samples were to be drawn at local labs and results sent to the clinical trial teams. Candidates would never have to visit a site at all, thus taking away many of the ease-of-access issues often cited as participation barriers. Patients were fully empowered to direct their trial participation, but were they fully engaged in this “clinical trial of the future” format?
By 2012, Pfizer announced that — while no less enthusiastic about incorporating social media and new technologies into the trial process — it was planning to wind the trial down after having disappointing online recruitment numbers. Was it a case of too much too fast? Were patients perhaps so “liberated” from the process that they ended up disengaged in a whole new way? Remember, The Neuropathy Association poll showed that interaction with leading experts was a key driver for trial participation. Was that element lacking? The lessons learned here are still being debated, but the market nonetheless commended the effort, and Pfizer announced it intends to try the virtual approach again very shortly, either here or abroad.10
ResearchMatch
Leveraging patient registries is receiving enormous focus as a critical building block for advancing research and improving trial enrollment numbers. One innovator bringing patients and investigators together in new ways by empowering patients and removing access barriers is ResearchMatch.org. Started in 2009, this online platform grew out of a grant to a local Vanderbilt University patient registry for innovating the process of connecting patients and investigators. Developed in partnership with consortia members and fully funded by NCATS, the platform takes advantage of new novel technologies, including those used by online dating sites like Match.com, to make connections in a secure and convenient environment. It allows patients and researchers to create their own online profiles, respectively, of themselves and of the ideal trial participant sought. This, then, allows the technology to “match” the two together in a blinded, progressive way that aids prequalification to increase match success rates.
Patient profiles do not have to be disease-specific and can include healthy individuals, thus enabling people to express their interest in different types of trials that might not have otherwise found them, like those addressing comorbidities. Researchers using the platform can target patient candidates in a much more directed fashion than available with previous recruitment efforts. Patients can take charge of their own access to trials and no longer have to wait for someone to tell them about a trial or struggle with doing online research. Instead, ResearchMatch helps investigators find them.
Originally only available to NIH-funded researchers, access has now been expanded to any nonprofit investigator in the U.S. and Puerto Rico. After just a few short years, ResearchMatch now hosts:
- 8,766 volunteers from 5,890 unique conditions and 832 rare conditions
- 4,169 pediatric volunteers
- 13 condition/disease-specific sub-registries (including six rare conditions)
- 3,000 researchers at 108 institutions
- 532 recruits in active studies
ResearchMatch project manager Catherine Gregor states, “ResearchMatch is challenging itself to constantly evolve to meet the needs of its community.” New additions and future plans include:
- Trial finder, launched in March 2015. Trial finder is a user-friendly interface with www.clinicaltrials.gov to find actively recruiting trials in a more consumer-friendly, searchable format. Patients can filter through trials and generate tabulated results with highlighted locations that can be printed or tabulated for easy sharing with others.
- Next will be an algorithm program for patients to scan PubMed for clinical trial articles.
- For investigators, a REDCap (Research Electronic Data Capture) partnership is in the works to enhance pre-screening surveys.
- And, the future holds an online consumer resource for information about completed trials pertaining to their interests and/or trials that they actively participated in. The application aims to keep volunteers engaged after a trial so they remain invested in the research process. “It’s for those interested in knowing ‘what did my contribution do?’” says Gregor.
Time Is Ticking
Indeed, this century has truly kicked off with a new era of patient engagement and patient-focused clinical trials. But, like developing the next blockbuster drug, time is of the essence, and the stakes are too high for not getting it right. Talking to all research stakeholders, one can almost hear the clock ticking — ticking off the lives holding out hope (and the lives lost), ticking off the years passing by and ticking off the dollars spent. Whether expressed directly or not, stakeholders’ frustration and even exasperation with each other and with the process is palpable. But one also senses the excitement and the optimism that new collaborations could yield extraordinary advances. Patients are claiming their place on the navigation team charting their future, and a journey of untold possibilities now lies ahead.
References
- Kish L. The Blockbuster Drug of the Century: An Engaged Patient. HL7 Health Standards, Aug. 28, 2012. Accessed at www.hl7standards.com/blog/2012/08/28/drug-of-the-century.
- Pharmaceutical Research and Manufacturers of America and Battelle Technology Partnership Practice. Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies. Accessed at www.phrma.org/sites/default/files/pdf/biopharmaceutical-industry-sponsoredclinical-trials-impact-on-state-economies.pdf.
- FasterCures. Accessed at www.fastercures.org.
- Coalition for Clinical Trial Awareness. PHRMA Did You Know? Clinical Trials: Advancing Medical Knowledge Fact Sheet. Accessed at www.cctawareness.org/wp-content/ uploads/2014/07/Did-You-Know_Clinical-Trials.pdf.
- Kousoulis AA. Clinical Trials in the Era of Big Data: Changing the Paradigm. Clinical Practice Research Datalink, July 14, 2014. Accessed at www.ccra.org.uk/Meetings/ 20140714/Downloads/1545_Dr_Antonis_Kousoulis.pdf.
- Patient-Centered Outcomes Research Institute (PCORI). About Us. Accessed at www.pcori.org/about-us.
- Patient-Centered Outcomes Research Institute (PCORI). PCORnet. Accessed at www.pcori.org/research-results/pcornet-national-patient-centered-clinical-research-network.
- Perfetto EM, Burke L, Oehrlein EM, and Epstein RS. Patient-Focused Drug Development-A New Direction for Collaboration. Medical Care, 53(1):13-14. January 2015.
- Tockarshewsky T. Talk presented at February 11-12, 2013, FDA Clinical Development Programs for Disease-Modifying Agents for Peripheral Neuropathy: Public Workshop Plenary Session. Neuropathy Clinical Trial Patient Participation Trends; Silver Spring, MD. 2013. Agenda available at www.fda.gov/downloads/Drugs/NewsEvents/UCM337927.pdf.
- Pfizer: We Won’t Abandon Social Media in Trials Because Virtual Study Failed. PMLive, June 21, 2012. Accessed at www.pmlive.com/pharma_news/pfizer_wont_abandon_social_media_trials_virtual_study_failed_408636.