Toward the Ultimate Cure: Gene Therapy for Severe Combined Immunodeficiency
- By Keith Berman, MPH, MBA
PRIOR TO THE successful use of hematopoietic stem cell transplantation (HSCT) to reconstitute the immune system, profound derangements of both cellular and humoral immunity in newborns born with severe combined immunodeficiency (SCID) were always fatal in infancy. Clinical experience accumulated over the last two decades has helped refine HSCT therapy and, thus, improve the odds of long-term survival for children born with this extremely rare genetic disorder. It is now apparent, for example, that for certain patients, the likelihood of long-term survival improves with a less intensive myeloablative conditioning regimen, or no conditioning at all. It’s now well-documented that survival odds are much better in SCID infants diagnosed and transplanted in the first three-and-a-half months after birth, or who are fortunate enough not to have experienced an infection prior to their procedure.1 This understanding of the importance of very early transplantation was a major impetus for universal newborn SCID screening with the T-cell excision circle (TREC) assay, which is now in place or is being implemented in 47 states.2
But the predominant factor that influences long-term survival is outside anyone’s control: the availability (or nonavailability) of blood-forming hematopoietic stem cells (HSCs) from an HLAidentical sibling donor. For SCID infants with a matched sibling bone marrow or peripheral blood stem cell donor, the prospects for long-term survival are now edging toward 100 percent.1 Unfortunately, less than 25 percent of patients have a matched sibling donor.3
SCID patients for whom only a matched unrelated or haploidentical HSC donor is available face far higher risks of life-threatening complications and death. Many continue to suffer severe recurrent infections due to only partial engraftment and incomplete restoration of immunity. HSCT will fail to adequately restore B-cell immunity in as many as one-half of these patients, necessitating chronic antibody replacement therapy with intravenous immune globulin (IVIG). Repeated breakthrough infections and complications of HSCT, including graft-versus-host disease, can cause cumulative damage to the lungs and other vital organs. Many of these children experience failure to thrive and cognitive deficits. More than one in four will succumb within the first five years after HSCT.1
The one remaining therapeutic option has been the dream of clinicians for decades: to treat the disorder by correcting it at its most fundamental genetic level, with gene therapy.
The Infancy of Gene Therapy
By the 1980s, genetic flaws causing the two most common forms of SCID had been identified and fully characterized:
- ADA-SCID: A defective gene-encoding adenosine deaminase (ADA) results in a deficiency of this metabolic enzyme, which is critical for lymphocyte differentiation and growth.
- SCID-X1: A defective gene on the X chromosome that encodes the common gamma chain of the interleukin-2 (IL-2) receptor (IL2RG) results in profound disruption of the development of T lymphocytes and natural killer (NK) cells.
In a handful of cutting-edge laboratories here and in Europe, techniques were developed to exploit the ability of gamma-retroviruses to ferry and insert normal copies of the affected genes directly into the DNA of CD34+ lymphocytes collected and purified from the patient’s own bone marrow or blood circulation. The idea was to expand these gene-corrected cells ex vivo and reinfuse them into the patient, to find their way into the bone marrow space and differentiate into the T and B lymphocytes and NK cells that mediate immune function (Figure 1).
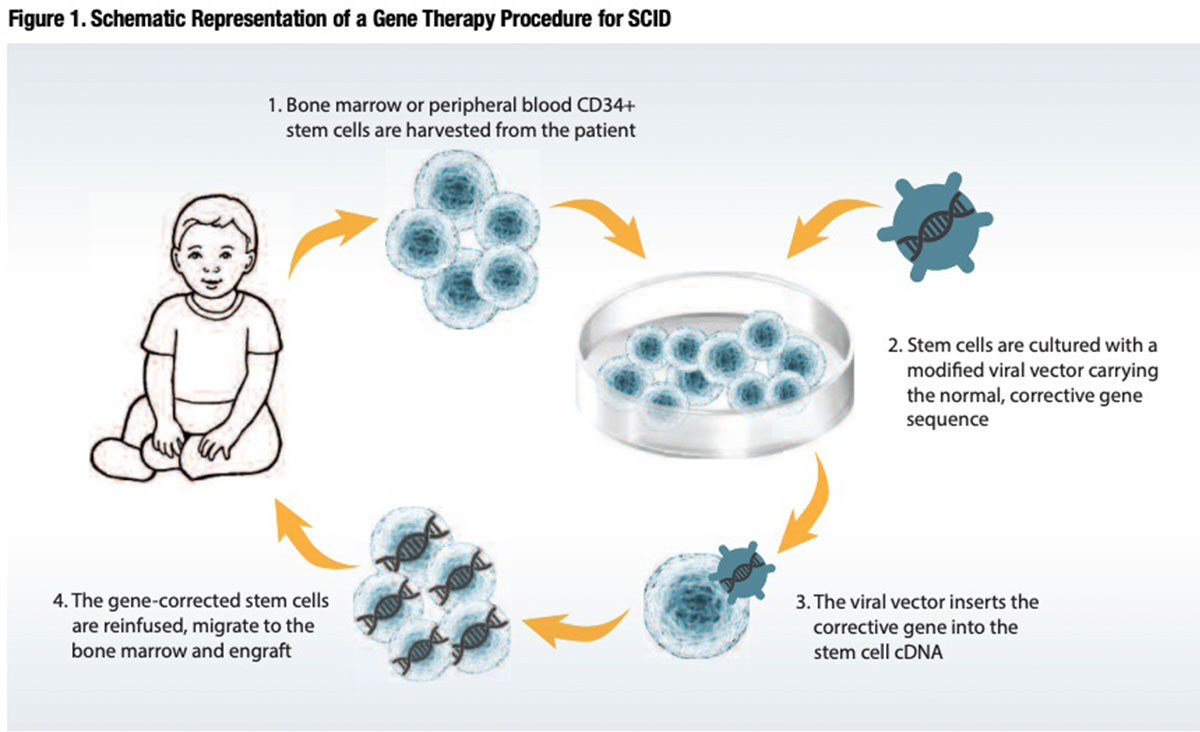
On Sept. 14, 1990, after much testing in animal models, U.S. investigators at the National Institutes of Health initiated the world’s first human gene therapy trial in a 4-year-old U.S. girl with ADASCID.4 Shortly thereafter, Italian investigators at the San Raffaele Telethon Institute for Gene Therapy initiated their own trial, employing different gamma-retrovirus vectors to insert the normal ADA gene into defective CD34+ lymphocytes harvested from patients with ADA-SCID.5
In simultaneous reports published five years later in the journal Science, the Italian and U.S. teams announced highly encouraging findings in a total of four ADA-SCID patients who had previously been supported with exogenous ADA replacement therapy.6,7 The T cell and NK cell counts normalized in all patients, as did a number of cellular and humoral immune responses. ADA gene expression persisted in the two U.S. patients at four-year follow-up. At that point, it appeared that the dream might soon become reality.
Protocol Change Transforms ADA-SCID Gene Therapy
Unfortunately, it soon became evident that the initial engraftment of retrovirus and ADA gene-transduced T lymphocytes did not sustain itself sufficiently over the long term; the small proportion of genetically corrected HSCs that did engraft failed to provide adequate protection against severe infections. Fortunately, later experimentation demonstrated that mild cytoreductive “conditioning” with low-dose busulfan or similar agents, essentially making room in the bone marrow for the reinfused gene-corrected CD34+ lymphocytes, was highly effective in facilitating immune reconstitution.
In 2002, the San Raffaele Telethon team was the first to describe sustained engraftment of genetically engineered HSCs using nonmyeloablative conditioning, with long-term increases in T-cell counts, normalization of T-cell function and restoration of a robust humoral response to vaccine challenges in two ADA-SCID patients.8 Seven years later, this same team reported the outcomes of gene therapy using autologous CD34+ bone marrow cells transduced with a gamma-retroviral vector in 10 children with ADA-SCID and no available HLA-identical sibling donor: All 10 patients were alive after a median of 4.0 years (range 1.8 to 8.0) with stable engraftment of HSCs. Nine of the 10 had normalization of T-cell function, and five no longer required IVIG replacement therapy. “Effective protection against infections and improvement in physical development has made a normal lifestyle possible,” the investigators reported.9 Other gene therapy research teams in Europe and the U.S. have published similar results with different ADA-SCID gene therapy protocols using a conditioning regimen and conventional gamma-retroviral vectors to insert normal copies of the ADA gene into CD34+ HSCs.10,11
New findings published this year by an international consortium again led by San Raffaele Telethon confirm 100 percent long-term survival in 18 consecutive ADA-SCID patients receiving gene therapy, with normalization of T-cell populations, reduced need for IVIG replacement and a 10-fold mean reduction in severe infection rates. 12 Further, in contrast to other severe primary immunodeficiency disorders treated with gene therapy, no cases of retrovirus-mediated insertional mutagenesis have been identified in any of the roughly 60 ADA-SCID patients treated to date.
A landmark event in May 2016 marked the culmination of a 25-year journey by these investigators, their clinical collaborators on three continents and the courageous families that agreed to participate. Acting on a recommendation by the European Medicines Agency — Europe’s equivalent of the U.S. Food and Drug Administration (FDA) — the European Commission approved Strimvelis (autologous CD34+ cells transduced to express ADA) for the treatment of patients with ADA-SCID for whom no suitable HLA-matched related stem cell donor is available. It is the world’s first licensed corrective childhood gene therapy.
Strimvelis will be marketed in European Union countries by GlaxoSmithKline, which also collaborated in its final stages of development. This individually customized treatment for ADA-SCID fulfills the promise of gene therapy: to essentially cure the more than three quarters of ADA-SCID children who do not have a suitable donor for HSCT.
Gene Therapy for SCID-X1: Full Stop to Full Speed Ahead
Concurrent with preliminary ADASCID trials in the early 1990s, other investigators were actively testing similar gene therapy protocols to treat male children with X-linked severe combined immunodeficiency (SCID-X1) and a poor HSCT prognosis. SCID-X1 is the predominant disease variant, accounting for 50 percent to 60 percent of all SCID cases. Preclinical studies confirmed that gamma-retroviral vectors effectively inserted the healthy gene for IL2RG into harvested CD34+ cells. A decade later, after incorporating the mild nonmyeloablative conditioning that produced durable engraftment in ADA-SCID gene therapy trials, small published SCID-X1 patient series documented persisting normalized T, B and NK cell counts and restored immune functions.13,14
But a shocking and unexpected setback put a halt to progress in SCID-X1 gene therapy: Five of thefirst 20 patients treated in these trials developed T-cell leukemia between two and five years after their procedure. In all cases, evidence pointed to aberrant activation of nearby oncogenes triggered by a powerful oncogene “enhancer” element within the gamma-retroviral vector.
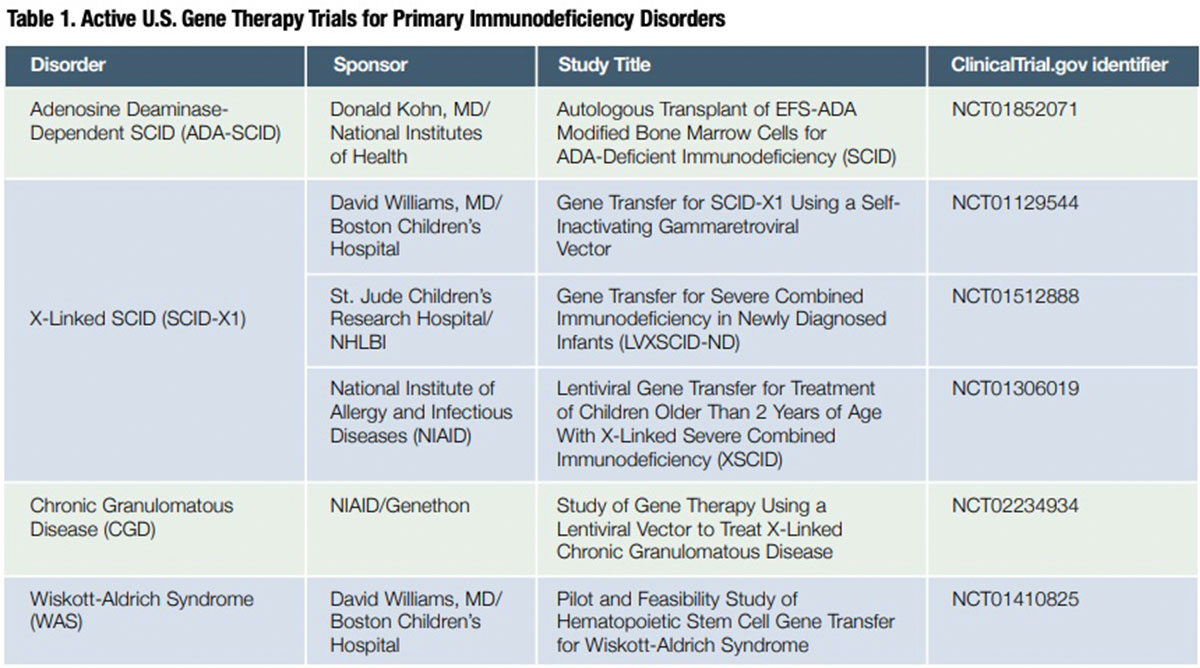
Investigators immediately set about developing safer gene transfer vectors. Two types are now being evaluated in clinical trials with encouraging efficacy results and, thus far, without any evidence of a leukemogenic effect. One is modified gamma-retrovirus vectors entirely devoid of enhancer sequences,15 and the second is novel “self-inactivating” lentiviral vectors designed to have a reduced risk of activating oncogenes.16 Extended patient accrual and follow-up will be necessary to establish whether these alternative vectors prove to be safe in current SCID-X1 gene therapy trials, as well as in ongoing trials evaluating gene therapy for two other primary immunodeficiency disorders — Wiskott Aldrich syndrome (WAS) and chronic granulomatous disease (CGD) — in which earlier use of gamma-retroviral vectors was also associated with fatal acute leukemia (Table 1).
On the Horizon: More Approvals for More Uses
Past challenges of long-term engraftment of gene-corrected progenitor cells and vector-associated leukemia risks appear to have been resolved. All evidence now suggests that gene therapy is largely curative for most patients with ADASCID and SCID-X1, without the risks of complications and graft failure associated with matched unrelated or haploidentical HSCT.
Additional findings from six currently ongoing gene therapy clinical trials 17 will be of value to further optimize patient outcomes. All evidence suggests that it is now only a matter of time before gene therapy protocols for these two rare genetic disorders — and potentially CGD, WAS and other life-threatening primary immunodeficiencies — secure FDA approval for commercialization.
Meanwhile, what has been learned about how to optimize the safety and efficacy of gene therapy from experience with SCID patients has helped researchers to design better gene therapy vectors and clinical protocols for far more common genetic disorders, including, for example, sickle cell disease, betathalassemia and hemophilia A and B. Thanks to this pioneering SCID research, thousands of patients with these debilitating disorders may not need to wait so long for potentially curative gene therapy: All three are currently the subjects of active clinical trials.
References
- Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med 2014 Jul 31;371(5):434-46.
- IDF SCID Newborn Screening Campaign (Immune Deficiency Foundation). Accessed 11/14/2016 at primaryimmune.org/idf-advocacycenter/idf-scid-newborn-screening-campaign.
- Cicalese MP, Ferrua F, Castagnaro L, et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 2016 Jul 7;128(1):45-54.
- Panno J. Gene Therapy: Treatments and Cures for Genetic Diseases. 2011: Facts on File, Inc.
- FerrariG, Rossini S, Giavazzi R, et al. An in vivo model of somatic cell gene therapy for human severe combined immunodeficiency. Science 1991 Mar 15;251(4999):1363-6.
- Blaese RM, Culver KW, Miller AD, et al. Tlymphocyte-directed gene therapy for ADA-SCID: initial trial results after 4 years. Science 1995 Oct 20; 270(5235):475.80.
- Bordignon C, Notarangelo LD, Nobili N, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients. Science 1995 Oct 20;270(5235):470-5.
- Aiuti A, Slavin S, Aker M,et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002 Jun 28;296(5577):2410-3.
- Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 2009 Jan 29;360(5):447-58.
- Gaspar HB, Cooray S, Gilmour KC, et al. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci TranslMed 2011 Aug 24;3(97)97ra80.
- Candotti F, ShawKL, Muul L, et al. Gene therapy for adenosine deaminase deficient severe combined immunedeficiency: clinical comparison of retroviral vectors and treatment plans. Blood 2012Nov 1;120(18):3635-46.
- Cicalese MP, Ferrua F, Castagnaro L, et al. Update on the safety and efficacy of retroviral gene therapy for immunodeficiency due to adenosine deaminase deficiency. Blood 2016 Jul 7;128(1):45-54.
- Cavazzana-Calvo M, Hacein-Bay S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000 Apr 28;288(5466):669-72.
- Gaspar HB, Parsley KL, Howe S, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gamma-retroviral vector. Lancet 2004 Dec 18-31;364(9452):2181-7.
- Hacein-Bey-Abina S, Pai SY, Gaspar HB, et al. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med 2014 Oct 9;371(15):1407-17.
- De Ravin SS, Wu X, Theobald N, et al. Lentiviral hematopoietic stem cell gene therapy for older patients with X-linked severe combined immunodeficiency. Blood 2015 Dec 3;126(23):261.
- Cicalese MP and Aiuti A. Clinical applications of gene therapy for primary immunodeficiencies. Human Gene Therapy 2015 Apr;26:210-9.