New Biosimilar Autoimmune Disease Treatment Approved by FDA

Celltrion’s Avtozma (tocilizumabanoh), a biosimilar to Actemra (tocilizumab), in both intravenous (IV)and subcutaneous (SC) formulations, has been approved by the U.S. Food and Drug Administration.
Fresenius Kabi’s Tyenne, a Biosimilar of Actemra, Is Approved to Treat Autoimmune Diseases

The U.S. Food and Drug Administration has approved Tyenne (tocilizumab-aazg), a biosimilar referencing tocilizumab (Actemra; Genentech), to treat multiple autoimmune diseases, including rheumatoid arthritis and juvenile idiopathic arthritis.
Third Humira Biosimilar Is Approved by FDA

FDA has approved Alvotech’s Simlandi (adalimumab-ryvk) as the third interchangeable Humira biosimilar.
FDA Approves Wezlana, First Interchangeable Biosimilar to Stelara
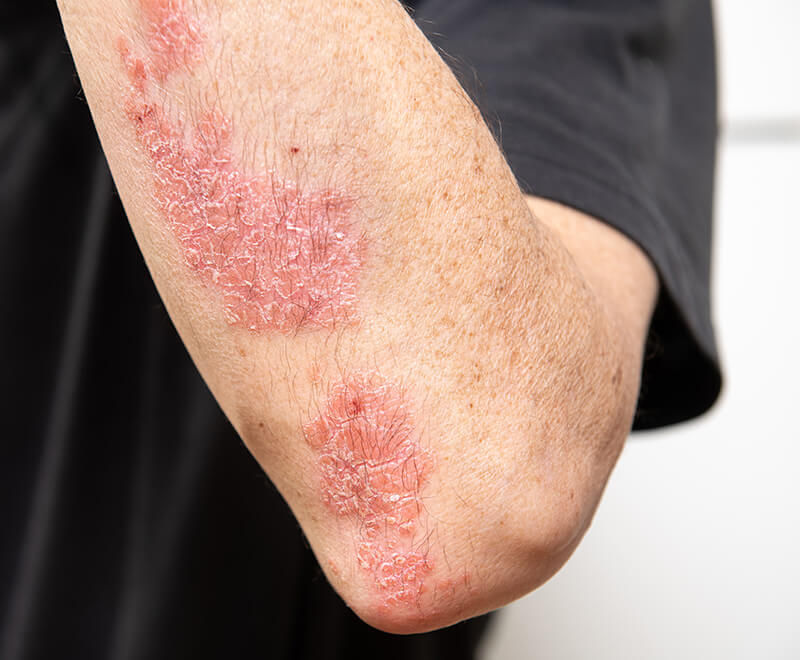
The U.S. Food and Drug Administration (FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara for adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy; active psoriatic arthritis; moderately to severely active Crohn’s disease; and moderately to severely active ulcerative colitis.
Biosimilars and the Fight Against Inflation

New legislation promised to increase patient access to critical drug products by lower costs. Will it work?
FDA Approves Humira Biosimilar to Treat Autoimmune Disorders
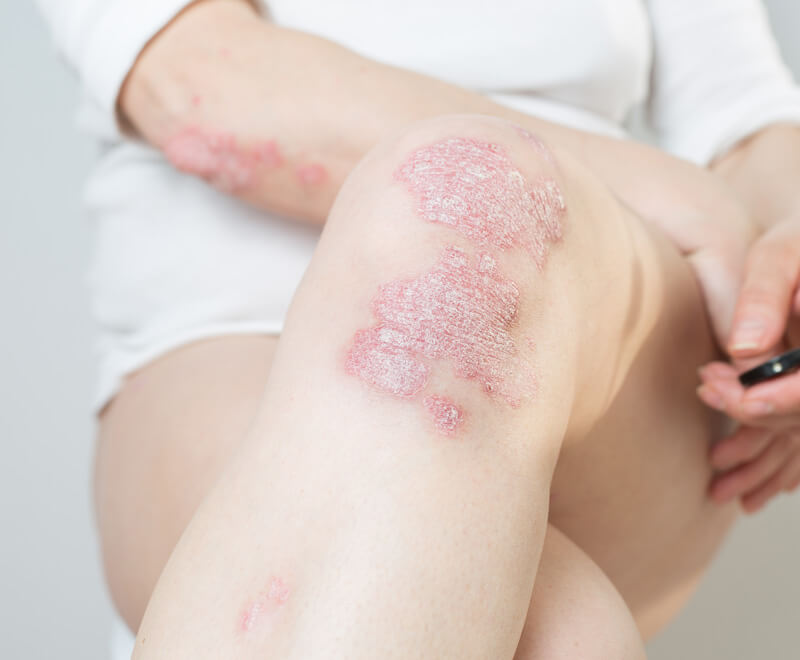
The U.S. Food and Drug Administration has approved Samsung Bioepis; Halima (adalimumab-bwwd) for the treatment of several autoimmune disorders, including rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and Crohn’s disease.
FDA Approves Biosimilar to Rheumatoid Arthritis Drug
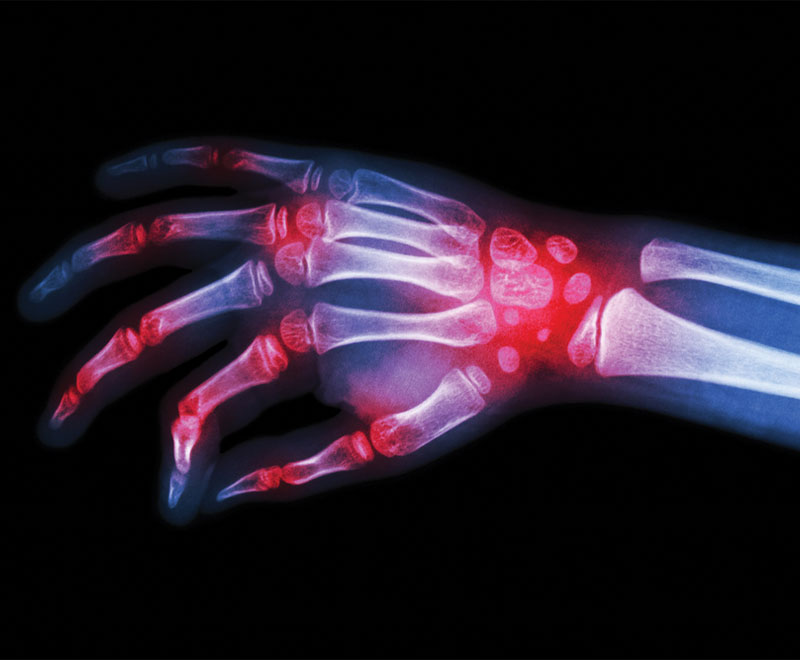
Amgen’s biosimilar to Johnson & Johnson’s rheumatoid arthritis drug, Remicade, has been approved by the U.S. Food and Drug Administration.
FDA Approves Sandoz’s Ziextenzo as 24th Biosimilar

The U.S. Food and Drug Administration (FDA) has approved Ziextenzo (pegfilgrastim-bmez), the 24th biosimilar approval in the U.S.
Nivestym, a Biosimilar to Neupogen, Approved by FDA
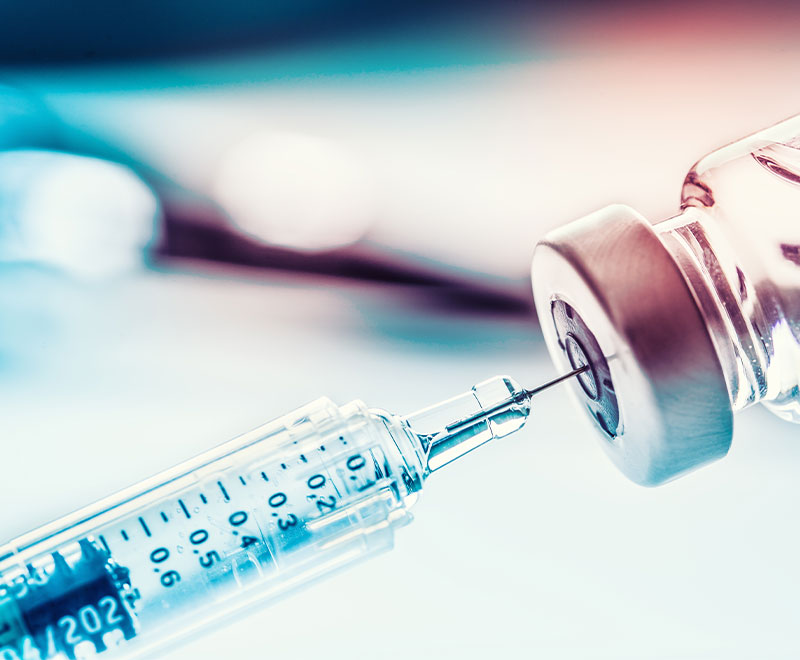
Pfizer’s Nivestym (filgrastim-aafi) has been approved by FDA for all eligible indications of the reference product.
Biosimilars: From Concept to Reality

Approvals in 2018 brought the total number of U.S. Food and Drug Administration-licensed biosimilars well into the double digits — and more are in the pipeline.
