HIV: A Patient’s Perspective
As a woman and activist living with HIV/AIDS, Maria Mejia’s mission is to give hope to the hopeless and send the message that she is far more than just a condition.
Myths & Facts: High Blood Pressure

Known as the silent killer, hypertension often goes undetected in individuals until a serious event occurs. However, with a better understanding of the condition and regular monitoring, it can be managed with treatment.
Update on Treating Neutropenia
While neutropenia can be a life-threatening condition, physicians have many tools to treat it.
New Study to Examine COVID-19 Vaccines in People with Weakened Immune Systems
Researchers at the University of Wisconsin (UW) School of Medicine and Public Health are exploring the ideal vaccine booster strategy for immunosuppressed patients to protect those at higher risk of severe illness and complications from COVID-19 infection.
CSL Behring Adds 4 and 5 Gram Vials of ZEMAIRA

CSL Behring’s ZEMAIRA (alpha1- proteinase inhibitor [human]) is now available in 4 gram and 5 gram vials.
World’s First Gene Therapies Approved to Treat Sickle Cell Disease
The U.S. Food and Drug Administration has approved two treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease in patients 12 years and older.
FDA Approves Repotrectinib for Non-Small Cell Lung Cancer
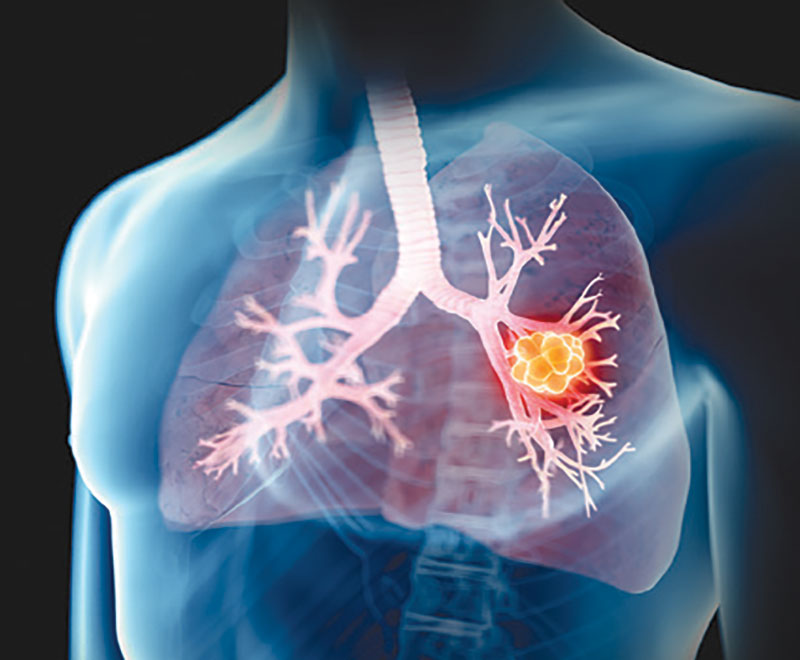
The U.S. Food and Drug Administration has approved Bristol Myers Squibb’s repotrectinib (Augtryo) for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer.
Fresenius Kabi’s Tyenne, a Biosimilar of Actemra, Is Approved to Treat Autoimmune Diseases

The U.S. Food and Drug Administration has approved Tyenne (tocilizumab-aazg), a biosimilar referencing tocilizumab (Actemra; Genentech), to treat multiple autoimmune diseases, including rheumatoid arthritis and juvenile idiopathic arthritis.
Selarsdi Approved as Biosimilar to Stelara to Treat Plaque Psoriasis and Psoriatic Arthritis
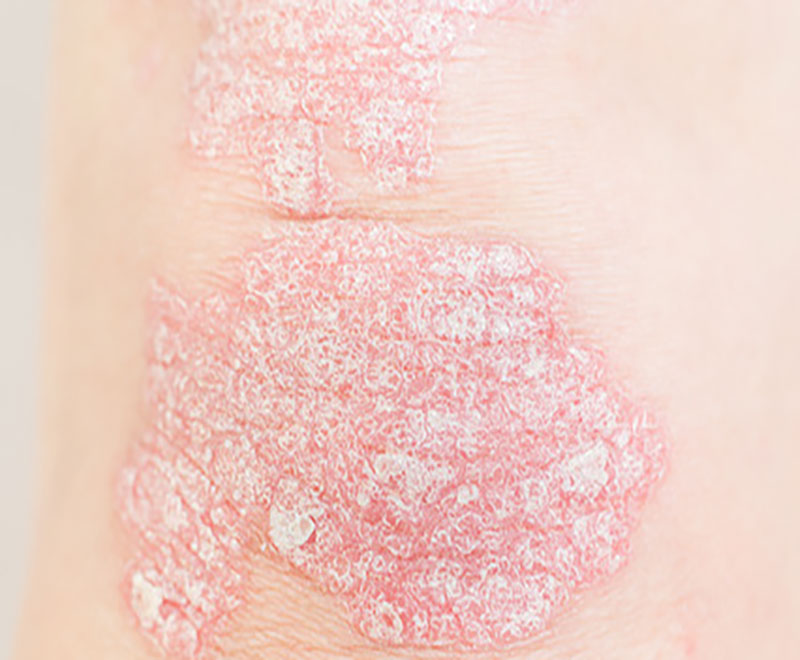
The U.S. Food and Drug Admini-stration has approved Selarsdi (ustekinumab-aekn) injection for subcutaneous use, as a biosimilar to Stelara, for the treatment of moderate to severe plaque psoriasis and for active psoriatic arthritis in adults and pediatric patients 6 years and older.
Blood Test Predicts Multiple Sclerosis Years Before Symptoms Appear
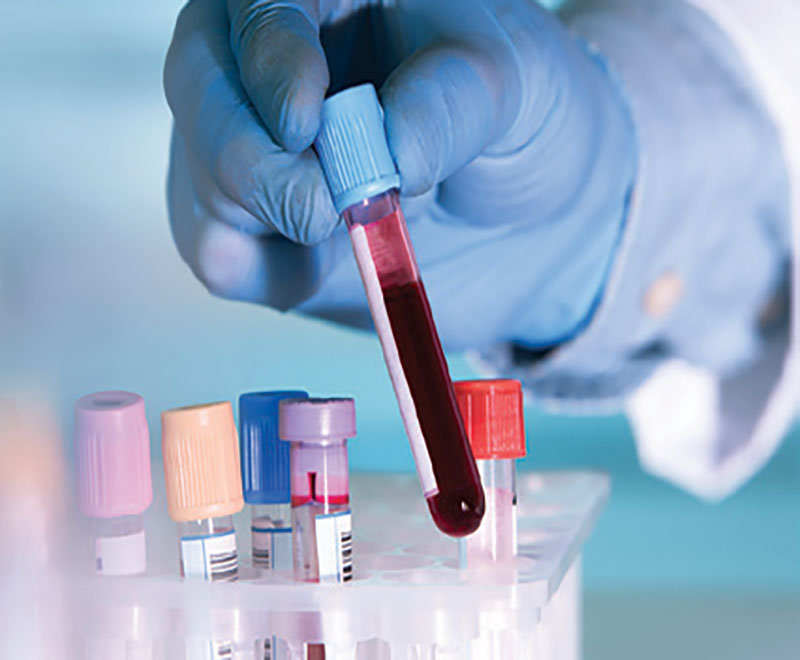
Researchers at the University of California, San Francisco, have identified a specific pattern of autoantibodies in the blood that precedes the clinical onset of multiple sclerosis.