GSK’s Penmenvy Meningococcal Vaccine Approved by FDA
GSK’s Penmenvy (meningococcal groups A, B, C, W and Y vaccine) has been approved by the U.S. Food and Drug Administration for use in individuals aged 10 through 25 years.
FDA Approves Dupixent for Chronic Obstructive Pulmonary Disease

The U.S. Food and Drug Administration (FDA) has expanded its approval of Dupixent to chronic obstructive pulmonary disease (COPD).
Updated COVID Vaccines Approved by FDA

FDA has approved and granted emergency use authorization for updated mRNA COVID-19 vaccines (2024-2025 formula) to include a monovalent (single) component that corresponds to the Omicron variant KP.2 strain of SARSCoV-2.
FDA Approves Epinephrine Nasal Spray
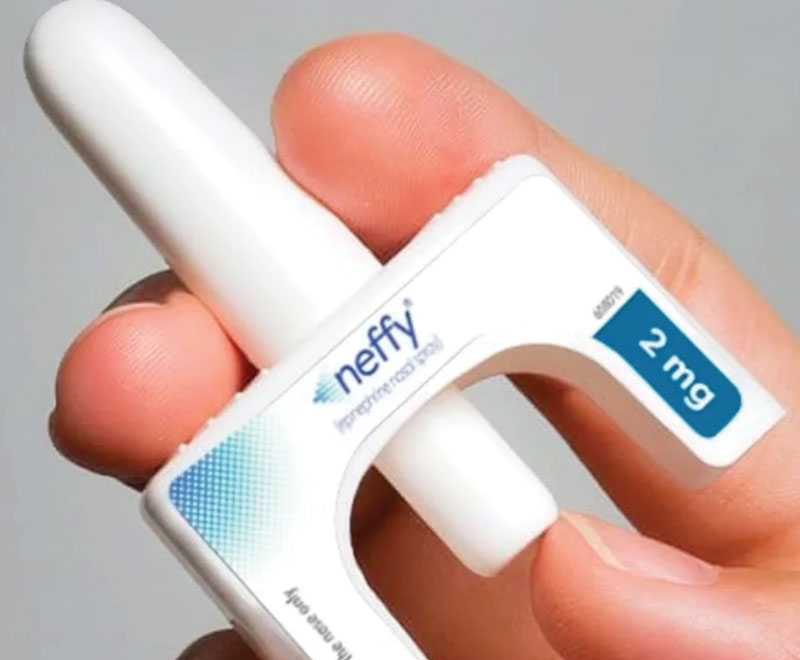
The U.S. Food and Drug Administration (FDA) has approved the first needle-free alternative to the EpiPen.
FDA Approves Additional Indication for Fibryga
FDA has approved Octapharma USA’s Fibryga (fibrinogen [human] lyophilized powder for reconstitution) for fibrinogen replacement in bleeding patients with acquired fibrinogen deficiency.
FDA Modernizes Informed Consent Guidance, Aligning with Common Rule Changes

FDA has published new draft guidance on informed consent that lines up with revisions to the Common Rule made in 2017, offering up-to-date recommendations on starting the process with the sharing of essential clinical trial information in ways that patients can understand.
FDA Retires Monovalent COVID-19 Vaccines

The U.S. Food and Drug Administration has amended the emergency use authorizations of the. Moderna and Pfizer-BioNTech COVID-19 bivalent mRNA vaccines to simplify the vaccination schedule for most individuals.
First Once-Daily Oral Plaque Psoriasis Drug Approved by FDA
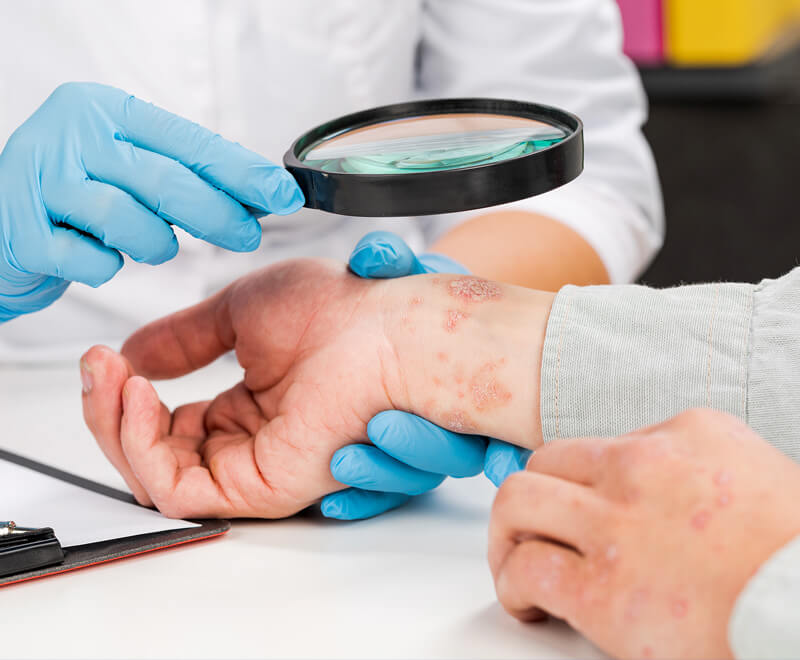
The U.S. Food and Drug Administration has approved Sotyktu (deucravacitinib), a once-daily oral pill by Bristol Myers Squibb, for adults who have plaque psoriasis (a chronic, systemic, immune-mediated disease) severe enough to make them candidates for systemic therapy and phototherapy.
FDA Approves Humira Biosimilar to Treat Autoimmune Disorders
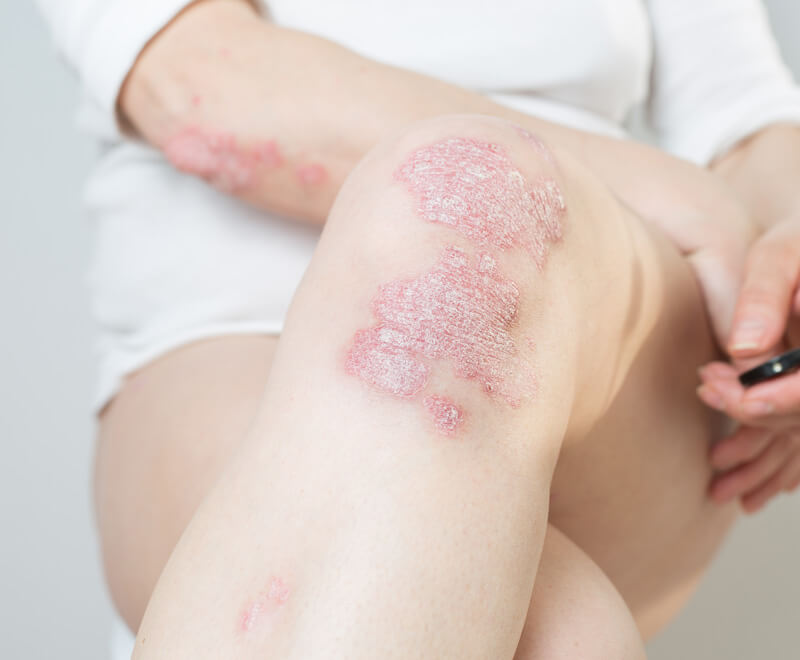
The U.S. Food and Drug Administration has approved Samsung Bioepis; Halima (adalimumab-bwwd) for the treatment of several autoimmune disorders, including rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and Crohn’s disease.
New Drugs Approved by FDA to Treat Eczema
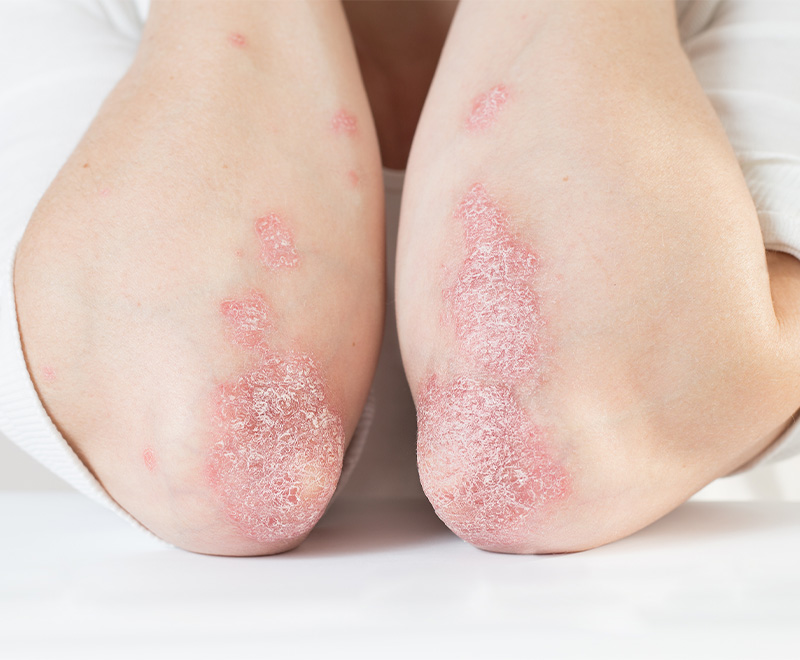
AbbVie’s Rinvoq and Pfizer’s Cibinqo have been approved by the U.S. Food and Drug Administration (FDA) to treat moderate-to-severe atopic dermatitis.
