Waiver Allows Medicare Patients to Receive Free Telehealth Services

The Centers for Medicare and Medicaid Services (CMS) has broadened access to Medicare telehealth services so beneficiaries can receive a wider range of services from their doctors without having to travel to a healthcare facility.
The Changing Face of Primary Care

How health systems and providers are adapting to new dynamics and demands in primary care.
FDA Issues Final Guidance on Biosimilar Interchangeability

The U.S. Food and Drug Administration (FDA) released guidelines on the studies companies need to conduct to show their biosimilar is interchangeable with a biologic.
New Voluntary Medicare Part D Demo Aims to Reduce Spending on High-Cost Drugs

CMS announced the Part D Payment Modernization Model to share in savings generated by reducing costs in the program’s catastrophic phase.
Medicare Costs Pose Rising Financial Burden on Older Adults

A recent study found that more than one-third of people with traditional Medicare spent at least 20 percent of their total income on out-of-pocket healthcare costs in 2013, and it is projected that number will increase to 42 percent by 2030.
HHS Proposed Significant Cuts to Medicare Payments for 340B Drugs

The U.S. Department of Health and Human Services has proposed 2018 updates to the Medicare hospital outpatient prospective payment system to decrease Medicare Part B payments to hospitals for 340B drugs by almost 30 percent.
Superbug Apocalypse: A Post-Antibiotic Era?
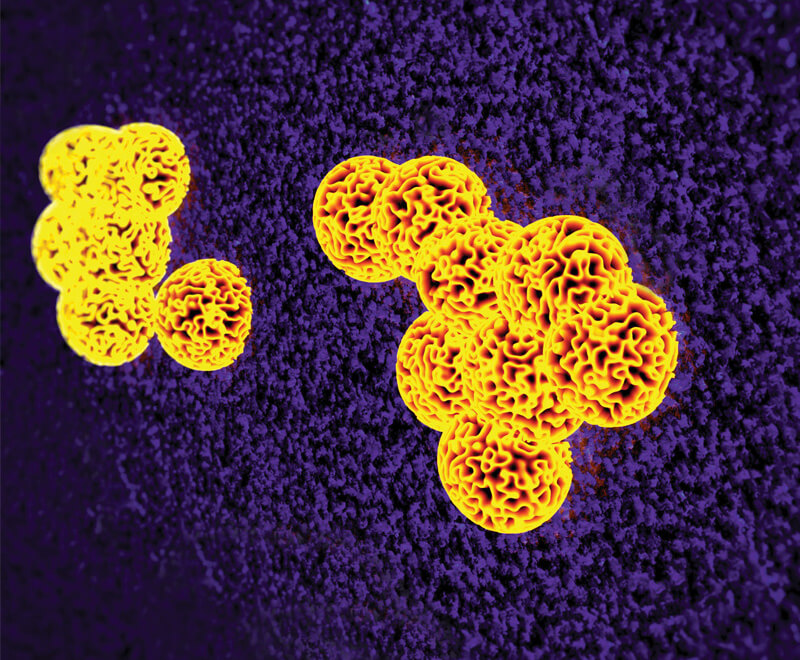
Antibiotics are the most commonly prescribed drugs worldwide. But now, widespread antimicrobial resistance caused by environments, hosts and overmedication may soon be the demise of these “wonder drugs.”
CMS Announces Biosimilars Reimbursement Rule

The Centers for Medicare and Medicaid Services issued final rules detailing how it will pay for services provided to Medicare beneficiaries in 2016.
New Law to Prohibit Medigap from Covering Part B Deductible

The Medicare Access and CHIP Reauthorization Act of 2015, which was enacted into law on April 16, will prohibit Medicare supplemental insurance(Medigap) policies from covering the Part B deductible for people who become eligible for Medicare on or afterJan. 1, 2020.
CMS Expands Coverage of HYQVIA for PI Patients to In-Home Use
The Centers for Medicare and Medicaid Services (CMS) has expanded coverage to include in-home use of HYQVIA(immune globulin infusion 10 percent [human] with recombinant humanhyaluronidase) to treat primary immunodeficiency patients.