COVID-19 Vaccine May Reduce Risk of Developing Long COVID

A new study published in the journal Vaccine, shows that receiving a COVID-19 vaccine could reduce the risk of developing long COVID if it’s administered five months before an infection.
FDA Approves YESINTEK to Treat Autoimmune Diseases

Biocon Biologics has received U.S. Food and Drug Administration (FDA) approval for YESINTEK, a monoclonal antibody for the treatment of autoimmune conditions, including Crohn’s disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis. Y
Study Reveals Best Time to Get RSV Vaccine During Pregnancy
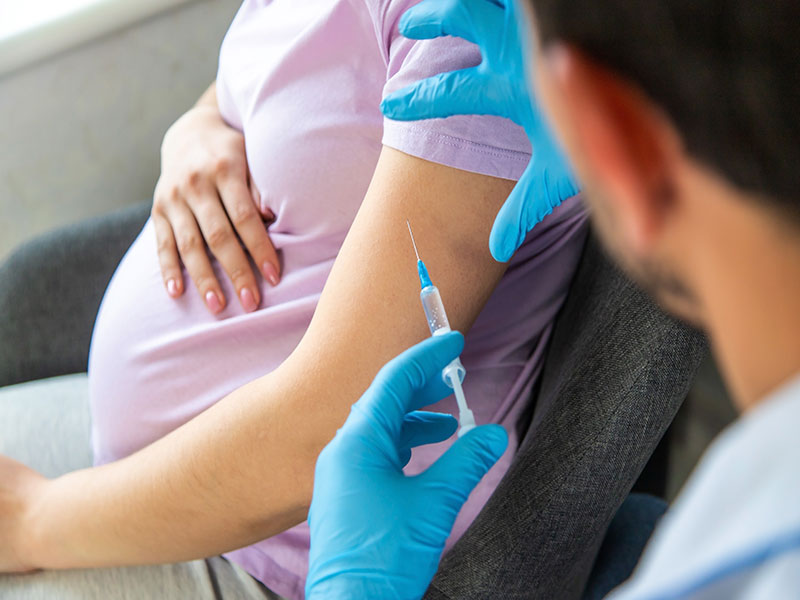
New research led by investigators at Mass General Brigham suggests vaccination closer to 32 weeks of pregnancy, rather than the current guidelines of between 32 and 36 weeks, will better protect pregnant women’s newborns against respiratory syncitial virus (RSV).
FDA Approves Nipocalimab to Treat Sjögren’s Disease

Johnson & Johnson’s anti-FcRn antibody nipocalimab is the first investigational therapy to be granted breakthrough therapy designation by the U.S. Food and Drug Administration as a treatment for adults with moderate-to-severe Sjögren’s disease.
Study Finds Link Between COVID-19 and Autoimmunity
A population-based study conducted by researchers from the Republic of Korea found a significantly higher risk of developing autoimmune and autoinflammatory conditions, such as rheumatoid arthritis (RA), lupus, Crohn’s disease, and alopecia, among individuals who had COVID-19, with risks particularly elevated for those with severe cases, Delta variant infections and those who were unvaccinated.
Nasal Swab May Predict Severity of COVID-19

New research suggests autoantibodies in the nasal cavity may predict the severity of COVID-19 disease.
IVIG Reduces Symptom Severity for AGID

A new study indicates intravenous immun globulin (IVIG) therapy can reduce symptom severity for patients with autoimmune gastrointestinal dysmotility (AGID), also known as gastroparesis.
mRNA Cancer Vaccine Begins Trials for Non-Small Cell Lung Cancer
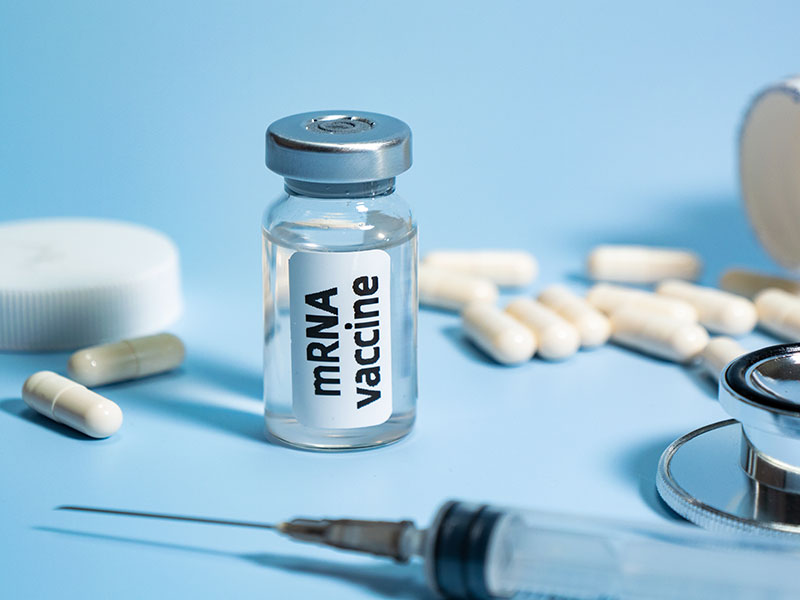
An mRNA vaccine has entered human trials as a treatment for lung cancer. Unlike traditional cancer vaccines such as the HPV vaccine, BNT116 is a therapeutic cancer vaccine designed to reduce tumor growth in patients with cancer or prevent its recurrence.
Changes in Immune Cell Gene Activity May Indicate Probability of Developing MS
A new study sheds like on the role of cytotoxic T cells in developing multiple sclerosis.
FDA Approves New Treatment for Hemophelia A and B
The U.S. Food and Drug Administration (FDA) approved Hympavzi (marstacimab-hncq) for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with hemophilia A without factor VIII inhibitors or hemophilia B without factor IX inhibitors (neutralizing antibodies).