FDA Approves and CDC Recommends Shingrix Vaccine to Prevent Shingles
GlaxoSmithKline (GSK) has receivedU.S. Food and Drug Administration approval for its Shingrix vaccine to prevent shingles (herpes zoster) in patients 50 years and older.
Subcutaneous Immunoglobulin Well-Tolerated and Effective in Mild to Moderate Exacerbations of Myasthenia Gravis

Canadian investigators at the University of Alberta evaluated the use of self-administered subcutaneous immune globulin in a prospective, open-label, Phase III crossover trial in adult patients with myasthenia gravis experiencing mild to moderate worsening of symptoms.
Kedrion Biopharma and Kamada Receive FDA Approval for KEDRAB
The U.S. Food and Drug Administration has approved Kedrion Biopharma’s and Kamada’s KEDRAB (rabies immune globulin [human]) for passive, transient postexposure prophylaxis of rabies infection.
NIH Grants $9 Million to Children’s Hospital Los Angeles for SCID Research
The National Institutes of Health’s National Institute of Allergy and Infectious Diseases division has awarded nearly $9 million to researchers from Children’s Center for Cancer and Blood Diseases at Children’s Hospital Los Angeles and Boston Children’s Hospital to study the lowest dose of chemotherapy needed for babies with severe combined immunodeficiency(SCID) undergoing bone marrow transplant, the standard treatment for SCID.
Cutting-Edge Pediatric Cancer Therapy Approved by FDA

Novartis’ Kymriah (tisagenlecleucel) has been approved by the U.S. Food and Drug Administration to treat pediatric acute lymphoblastic leukemia.
Supreme Court Rules in Favor of Faith-Based Hospitals’ Exemption from ERISA

The U.S. Supreme Court ruled that faith-based hospitals’ pension plans qualify for the “church plan” exemption from the Employee Retirement Income Security Act (ERISA).
FDA Approves First Drug to Treat Giant Cell Arteritis
The U.S. Food and Drug Administration has approved Roche’s Actemra (tocilizumab), the first treatment for adult patients with giant cell arteritis.
Octapharma USA’s NUWIQ Receives FDA Approval for Expanded Vial Strengths

The U.S. Food and Drug Administration has approved new product strengths for Octapharma’s NUWIQ.
Recombinant Quadrivalent Flu Vaccine More Effective in Older Adults
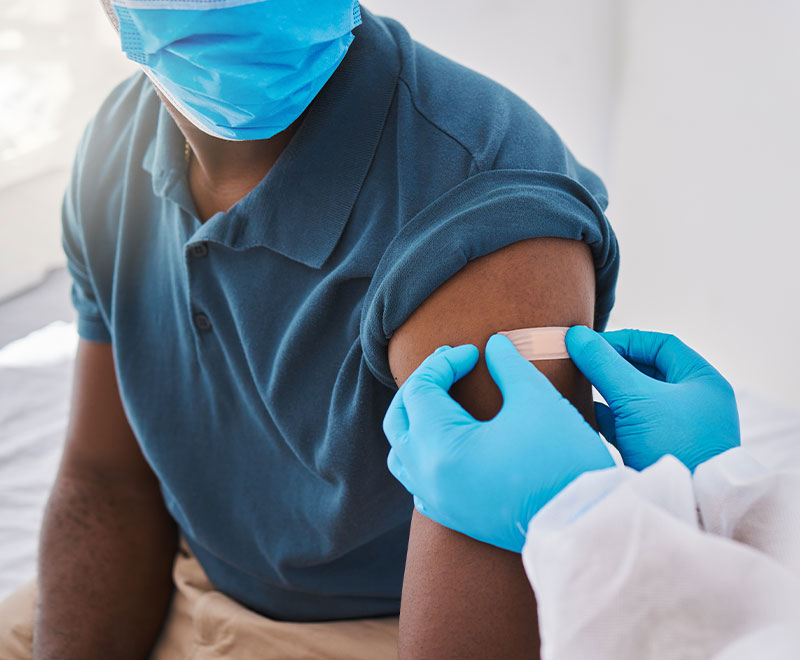
A clinical trial comparing the protective efficacy in older adults of a quadrivalent recombinant influenza vaccine with a standard-dose, egg-grown quadrivalent inactivated influenza vaccine during the A/H3N2-predominant 2014-2015 influenza season showed RIV4 provided better protection against confirmed influenza-like illness among older adults.
More Small Practices Exempt from MACRA Under New Draft Rule

The Centers for Medicare and Medicaid would exempt physician practices with less than $90,000 in Medicare revenue or fewer than 200 unique Medicare patients per year from complying with the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.