Report Shows Hospital-Acquired Conditions Continue to Decline

The Agency for Healthcare Research and Quality has released a preliminary update to the National Scorecard on Rates ofHospital-Acquired Conditions that show a 21 percent reduction in hospital-acquired conditions from 2010 to 2015.
Sanofi Pasteur Acquires Protein Sciences

Sanofi Pasteur has acquired Protein Sciences, a privately held vaccines biotechnology company based inMeriden, Conn.
Study Shows Microneedle Patch Influenza Vaccine Is Effective and Painless
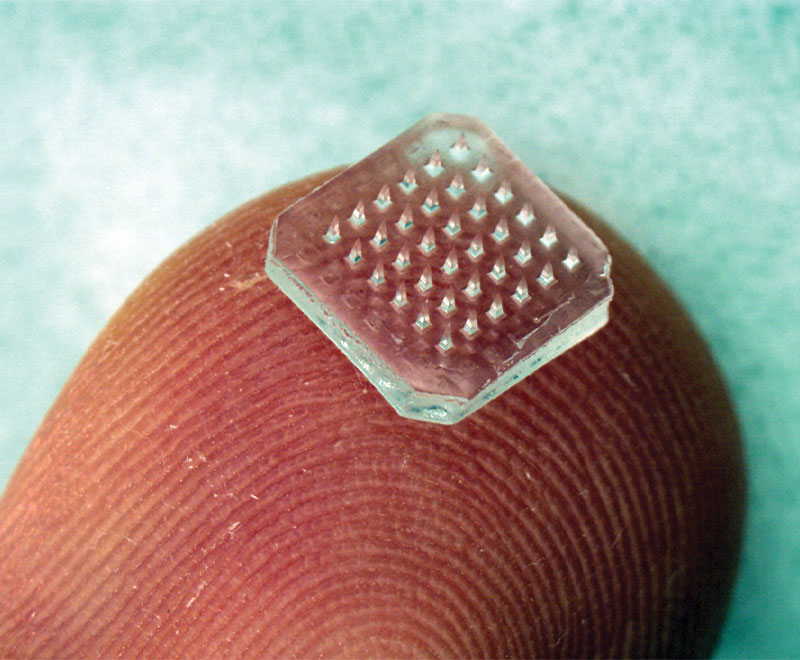
A new study shows that microneedle patches provide an alternative to conventional needle-and-syringe immunization, potentially offering improved immunogenicity, simplicity, cost-effectiveness, acceptability and safety.
Co-Administration of Albumin with Lactulose Reverses Hepatic Encephalopathy and Reduces Mortality in Liver Cirrhosis

A multidisciplinary team of investigators based in New Delhi randomized 120 patients with liver cirrhosis and overt hepatic encephalopathy (HE) to receive oral lactulose therapy or oral lactulose plus 1.5 g/kg/day of human albumin. The primary study endpoint was complete reversal of HE; secondary endpoints included mortality and length of hospital stay.
Public Attitudes Toward Unvaccinated Children and Their Mothers Depends on Motivation Not to Vaccinate

A study conducted at the University of British Columbia found that mothers are viewed negatively if their child hasn’t been vaccinated, regardless of the reason.
NIH Awards Grant to Study the Connection Between Autism and Autoimmunity During Pregnancy

The National Institutes of Health has awarded a $3 million grant to Feinstein Institute for Medical Research scientists to study the relationship between a mother’s autoimmunity during pregnancy and the risk of autism spectrum disorder in her child.
New Study Supports CDC Recommendation for Two-Dose HPV Vaccine in Children
A new study conducted at the Boston Medical Center is the first to support new recommendations for a two-dose HPV vaccine to prevent genital warts in kids younger than 15 years old.
HHS Renames AIDS.gov to HIV.gov

The U.S. Department of Health and Human Services has officially changed the name of AIDS.gov, the federal government’s source for information about HIV, to HIV.gov.
ADMA Biologics Acquires Biotest’s Therapy Business Unit

ADMA Biologics, which develops, manufactures and commercializes specialty plasma-based biologics for the treatment of immune deficiencies and prevention of certain infectious diseases, has acquired Biotest Pharmaceuticals’ Therapy Business Unit.
IVIG Highly Protective in Ferrets After Challenge with Severe Pandemic pH1N1 and Avian H5N1 Influenza Strains
Australian investigators tested the ability of intravenous immune globulin to protect against potential pandemic influenza virus in outbred ferrets, which are naturally susceptible to human influenza viruses and considered a relevant small animal model of human influenza infection.