The Growing Scourge of Counterfeit Pharmaceuticals
Counterfeits pose a threat for healthcare professionals worldwide. And, while technological advancements are being made to protect the drug supply, international regulation and politics stand in the way of fixing the problem in the foreseeable future.
- By Jim Trageser
One of the defining characteristics of modern medicine is the absolute trust both patients and providers place in the delivery system. From ambulance to admission, diagnosis to treatment, Western medicine has developed into such a forthright and upstanding infrastructure that we simply accept at face value the entire medical care chain — allowing physicians to focus their energy and time on dealing with whatever medical issues brought patients into their care in the first place.
However, the growing problem of counterfeit pharmaceuticals threatens this trust. Worse, it puts at risk the well-being, health and very lives of those exposed to these fraudulent products. It also potentially exposes physicians to liability — even though they may have accurately diagnosed the patient, prescribed the appropriate remedy and acted in good faith throughout.
Currently, counterfeit pharmaceuticals are a major problem mostly in developing areas of the world.1 But with many physicians from developed nations volunteering their time and skills to serve people in the developing world through groups like Doctors Without Borders and many churches, the issue of counterfeit drugs is one that affects all doctors. And the threat of counterfeit prescriptions in Western nations is growing; the U.S. Food and Drug Administration (FDA) reported in February that a counterfeit batch of a cancer drug was discovered in the United States.2
Counterfeiting is a threat in all delivery routes. While a recent study from St. Thomas’ Hospital in London indicated that the traditional supply route is less vulnerable to counterfeit infiltration than online purchases made directly by patients,3 even that is not immune to being compromised.4
What Are Counterfeit Drugs?
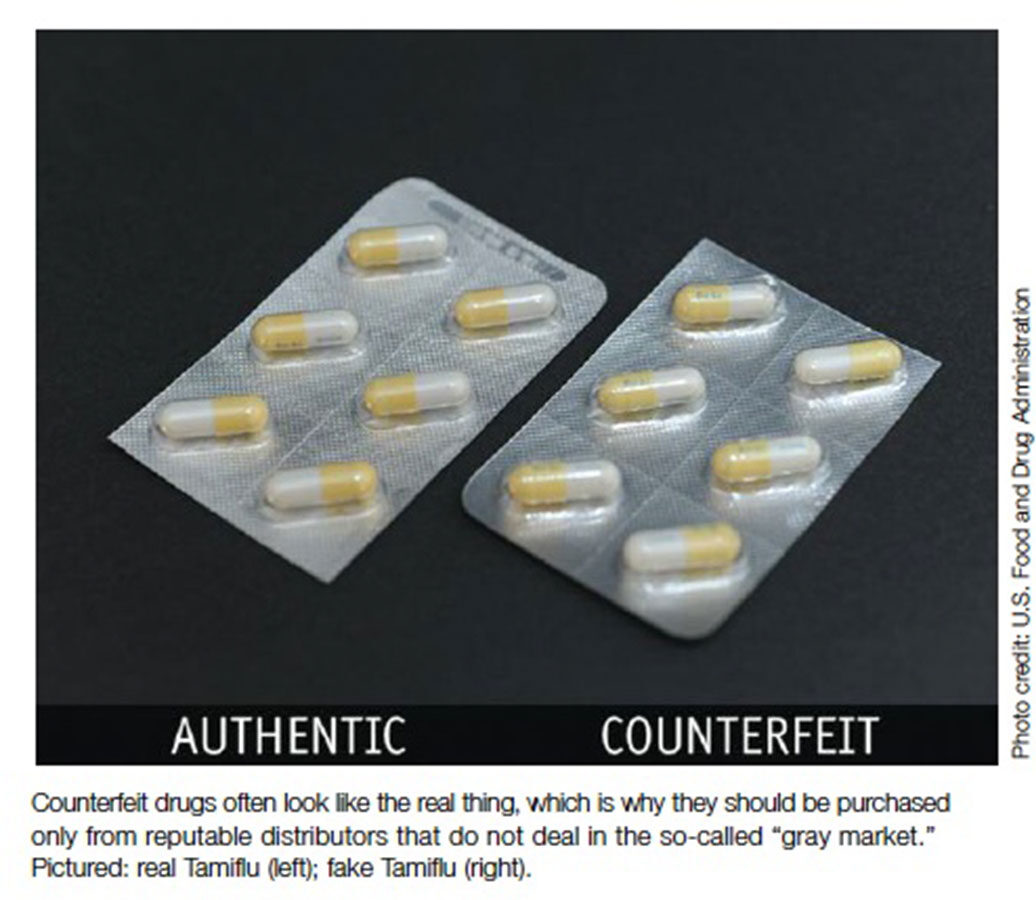
Counterfeits are imitations of legitimate drugs, manufactured and packaged to look like the real thing and passed off to consumers as legitimate. Counterfeits generally are not chemically identical to the legitimate drug; they may contain harmful ingredients, or they may pose no health risk of their own but lack the active ingredients to combat whatever disease or condition the physician is treating. Other times, they contain the wrong dosages of the active ingredient or ingredients. As with other counterfeit goods, counterfeit drugs will be manufactured to look as close to the legitimate product as possible.5 Indeed, the packaging and product may appear identical.6
It is important to note that counterfeit drugs are not generics. Generic drugs — low-cost versions of brand-name patented prescriptions —are subject to the same testing and regulation as the brand-name versions. In addition, counterfeit pharmaceuticals are not manufactured to compete with brand-name drugs the way generics are. They are a fraudulent criminal enterprise whose only purpose is to pass as a legitimate product. They undergo no testing, and those manufacturing them purposely avoid any regulation or oversight.
Who Makes Counterfeit Drugs?
Given the criminal nature of counterfeit drugs, it is impossible to say with any precision who is behind their manufacture and distribution. However, law enforcement officers from around the world believe that the same organized crime outfits that deal in other counterfeit goods are behind the growing counterfeit pharmaceuticals: the Mafia and other crime families, and even terrorist groups looking to finance their operations.7 The pharmaceutical firm Pfizer reports that the profits from counterfeit prescriptions are often greater than those for street drugs like heroin or cocaine.8
Many counterfeit pharmaceuticals can be traced to China and India.9 Other reports show that Latin America is a source of faked drugs.10 As with any organized criminal enterprise, those spots around the world with a weak government and spotty law enforcement are most likely to host drug counterfeiters.
Infiltrating the Supply System
Of course, manufacturing a fake pill that looks almost exactly like a popular brand-name drug won’t generate any money in and of itself. Money is made when there is a demand for scarce drugs or when there is a demand to pay less for more costly drugs.
In developing nations with little government oversight, inserting counterfeit pharmaceuticals into the supply chain is fairly simple. The World Health Organization estimates that some 64 percent of anti-malarial drugs in Nigeria are counterfeit.11
And those patients in the West who purchase prescription drugs online open themselves up to the risk of inadvertently purchasing counterfeits. A recent article in the Chicago Tribune pointed out that when consumers buy from an unlicensed, overseas online pharmacy, they have no guarantee that the drugs they receive are what their packaging claims. That same article, though, also listed several recent breaches of the domestic U.S. pharmaceutical supply system — breaches in which respected national drugstore chains and some physicians in northern Illinois were victimized by prescription drug counterfeiters.12
Pfizer’s brochure on counterfeiting warns that prescription drug wholesalers represent a weak link in the distribution chain, the point where counterfeit drugs can infiltrate the supply.8 However, the physicians in northern Illinois had apparently dealt with an overseas provider. Either way, both examples are sobering reminders that the amount of money in legitimate pharmaceuticals — well into the hundreds of billions of dollars per year13 — is attracting hardened and sophisticated criminal enterprises with little compunction about targeting doctors or the poor with their bogus products.
Fighting Back
The rising tide of faked prescription drugs is being addressed by both government agencies and legitimate pharmaceutical companies. With patient health and lives at risk, governments have an obvious interest in intervening. But with some $75 billion a year going to counterfeiters,14 the pharmaceutical industry is realizing that it, too, needs to be proactive.
Governments are responding to the problem locally through stepped-up enforcement and new legislation, and globally with new treaty proposals and international agreements to stem the flow of counterfeit drugs across borders. And, while the pharmaceutical companies whose products are being counterfeited support many of the governmental approaches, they also are pushing for technological tools to better fight counterfeiting immediately. From lab tests that can detect counterfeits to controlled tracking of legitimate prescription drugs from manufacture to point of sale, new technology can at least make it more difficult and more expensive for counterfeiters to ply their illicit trade.
Updating the Law
The largest obstacle to cracking down on fake pharmaceuticals is the wide disagreement in national laws that regulate the issue, as well as the lack of international consensus — not only about how to tackle counterfeits, but also about the more central question of how to define counterfeits. When the problem of counterfeit pharmaceuticals was first discussed at a World Health Organization (WHO) conference in 1985,15 existing trade and intellectual property laws and treaties were used to approach the problem. In the years since, those preexisting laws and treaties have proved ineffective at stemming counterfeit pharmaceuticals.
Further complicating the legal landscape, ongoing disagreements about how to define (and ban) counterfeit drugs while allowing for the development of low-cost but legitimate generics often involve competing national interests among developing and developed countries — disputes that have prevented a unified front in the fight against purely criminal counterfeits.16 The governments of many developing nations portray efforts to crack down on counterfeits as nothing more than international bullying, accusing the U.S. and other Western nations of being more interested in protecting the profits of large pharmaceutical companies than in providing affordable care to poor people who can’t afford patent-protected brand-name drugs.17
This ongoing disagreement over counterfeits versus generics has led to a situation in which there is no globally accepted standard of what constitutes a counterfeit, and so legal enforcement is a hodgepodge. Each nation has its own laws on trademark and copyright and its own web of treaties with other nations determining how complaints from abroad will be handled. The inability to craft agreement on just what constitutes an illegal counterfeit quite obviously makes it difficult to crack down on fake pharmaceuticals.
Promising Advances
If the law is years away from providing an internationally agreed-upon standard of what constitutes a counterfeit drug, technology is beginning to offer some tools for physicians, pharmacists and local governments to determine if a drug’s contents match the labeling. And the promise of more such tools are on the horizon.
Thermo Scientific has developed handheld spectrometer scanners that can authenticate the legitimacy of pharmaceuticals at any point in the supply chain.18 By comparing the spectrum signature of a pill or liquid against the known signature of legitimate pharmaceuticals, the scanner can detect counterfeits (or even bad batches of legitimate drugs that may not be effective due to poor quality control).
In Europe, a Swiss collaboration of academics and hospitals produced the Budget Capillary Electrophoresis, or ECB, which can quickly (in roughly 20 minutes) identify 80 percent of the 200 or so “core” medicines listed by the WHO by measuring how quickly they move through a capillary system and comparing that to known precise measurements for the legitimate drugs.1
Other pharmaceutical verification testing products are in the development stage, with technologies ranging from near-infrared spectroscopy to near-infrared chemical imaging. In each case, the test either scans with a light beam and measures the spectrum or uses a small sample for chemical analysis to compare the results against known values. If the measurement doesn’t match the known value, the sample will be flagged.
Another technological approach is secure packaging in which each retail unit is embedded with a high-quality three-dimensional holographic image. DuPont’s Izon technology19 increases the difficulty counterfeiters have in making their bogus drugs look exactly like the legitimate products they are targeting, and also lowers the potential profit by significantly increasing the cost of package mimicry.
While these new technological innovations are widely available in the West, a new study from the Institute of Medicine of the National Academies in Washington, D.C., points out that few agencies, public or private, in the developing nations most in need of these new tools have the ability to pay for them.20
Technology Meets the Law
But none of these technologies can be effective in protecting the domestic prescription drug supply chain if there are not industrywide protocols in place to ensure that the supply is tested repeatedly and robustly to detect and remove counterfeited products.One such protocol is an e-pedigree. An e-pedigree is an electronic tag that tracks a specific product from manufacture to final retail sale. Unfortunately, at this time, the use of e-pedigrees is spotty and uneven globally, and even varies state to state in the U.S. While California and Florida have taken the lead in implementing e-pedigree standards for pharmaceuticals (with industry adoption mandated to begin as soon as 2015), political opposition from supply chain companies, among others, has kept Congress or the FDA from implementing a national standard.21 The original proposed start date of 2015 has been pushed back significantly in most other states that have adopted an e-pedigree rule.
Protecting Yourself and Your Patients
The FDA’s Counterfeit Medicine web page lists more than a dozen prominent counterfeit scams in the United States in just the past few years.22 With that northern Illinois group of doctors facing FDA investigation for using counterfeit cancer drugs purchased from an overseas supplier, it’s clear that physicians will have to be more aware of the source of their pharmaceuticals moving forward. As the FDA pointed out in a recent safety warning to healthcare professionals: “If a medication’s price sounds too good to be true, it can be a sign the drugs offered are substandard, unapproved, stolen or counterfeit. Purchasers should be wary of deep discounts on expensive drugs.”23
When dispensing pharmaceuticals from a practice, it is imperative that physicians use the best practices they are already familiar with: Purchase only from reputable distributors that do not deal in the so-called “gray market” — overseas secondary wholesalers that buy and sell from one another, and may even repackage the products. This is the weak point in the distribution chain in Western countries.
There is even more physicians and other healthcare professionals can do to protect their patients from counterfeit pharmaceuticals. When not providing a drug directly, but prescribing a controlled substance for patients, they can emphasize the importance of filling that prescription through a reputable local pharmacy. If patients are concerned about the costs, particularly of maintenance pharmaceuticals, physicians can work with them to identify low-cost generic alternatives. If no generic alternative exists, there may be a nonprofit organization to which physicians can refer them that is dedicated to the cause of affordable medication in the community.24
Patients themselves remain perhaps the best indicator of the presence of counterfeit drugs in the supply chain. The National Association of Boards of Pharmacy points out that patients are most likely to notice if a prescription tastes different than previously or if the texture or color has changed. They also will notice side effects or a lack of efficacy.25 Physicians can and should counsel their patients on the dangers of counterfeit pharmaceuticals, and advise them to immediately report any concerns if they notice any of the above signs.26
A Continuing Problem
The problem of counterfeit pharmaceuticals is not going away any time soon, and for those of us in the West, it is likely to get worse before it gets better. As with all medical issues, it will be physicians who play a large role in how the problem is addressed — and how patients protect themselves in the here and now.
References
- Mombelli A. Swiss Technology Battles Fake Drugs. International Service of the Swiss Broadcasting Corp., Dec. 28, 2012. Accessed at www.swissinfo.ch/eng/science_technology/Swiss_technology_battles_fake_drugs.html?cid=34595272.
- U.S. Food and Drug Administration. Health Care Provider Alert: Another Counterfeit Cancer Medicine Found in United States. Feb. 5, 2013. Accessed at www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/CounterfeitMedicine/ucm338283.htm.
- Jackson G, Patel S, Khan S. Assessing the problem of counterfeit medications in the United Kingdom. Int J Clin Pract 2012 Mar;66(3):241-50. doi: 10.1111/j.1742-1241.2011.02826.x. Epub 2011 Nov 9. Accessed at www.ncbi.nlm.nih.gov/pubmed/22070229.
- Goralski MA, LeBlanc HP, Adams MG. Problems of Counterfeit International Pharmaceutical Products. Business Research Yearbook, 2011. Accessed at blue.utb.edu/lfalk/BRY2011v1.pdf#page=146.
- Charatan, F. Fake prescription drugs are flooding the United States. BMJ 2001 June 16; 322(7300): 1446. Accessed at www.ncbi.nlm.nih.gov/pmc/articles/PMC1173338.
- U.S. Food and Drug Administration. Counterfeit Drugs Flickr page. Accessed at www.flickr.com/photos/fdaphotos/sets/72157625426153869.
- Finlay BD. Counterfeit Drugs and National Security. Stimson, February 2011. Accessed at www.stimson.org/images/uploads/research-pdfs/Full_-_Counterfeit_Drugs_and_National_Security.pdf.
- Counterfeit Pharmaceuticals: A Serious Threat to Patient Safety. Pfizer Inc., 2007. Accessed at www.pfizer.com/files/products/CounterfeitBrochure.pdf.
- McLaughlin KE. Counterfeit medicine from Asia threatens lives in Africa. The Guardian, Dec. 23, 2012. Accessed at www.guardian.co.uk/world/2012/dec/23/africa-counterfeitmedicines-trade.
- What’s in That Pill? Bloomberg Business Week, June 17, 2001. Accessed at www.businessweek.com/stories/2001-06-17/whats-in-that-pill.
- Bad Medicine. The Economist, Oct. 13, 2012. Accessed at www.economist.com/node/21564546.
- Shelton DL. Fake medicine poses growing threat to consumers. Chicago Tribune, Oct. 16, 2012. Accessed at articles.chicagotribune.com/2012-10-16/business/ct-met-online-pharmacy-fakedrugs-20121016_1_counterfeit-drugs-illegal-online-pharmacies-illegal-medicine.
- Wikipedia.Pharmaceutical Industry. Accessed at en.wikipedia.org/wiki/Pharmaceutical_industry#Industry_revenues.
- The Painful Costs of Counterfeit Prescription Drugs. CNBC.com, Oct. 4, 2011. Accessed at www.cnbc.com/id/44759614/The_Painful_Costs_of_Counterfeit_Prescription_Drugs.
- World Health Organization. General Information on Counterfeit Medicines. Accessed at www.who/int/medicines/services/counterfeit/overview/en.
- WHO: Approach to “Counterfeit” Drugs May Affect Access to Medicines. Third World Network. Accessed at twnside.org.sg/title2/wto.info/2009/twninfo20090101.htm.
- Mara K. Counterfeit Medicines in WTO Dispute Process, Heating Up at WHO. Intellectual Property Watch, May 12, 2010. Accessed at www.ip-watch.org/2010/05/12/counterfeitmedicines-in-wto-dispute-process-heating-up-at-who.
- Rapid Detection of Counterfeit Materials. Thermo Scientific. Accessed at www.ahurascientific.com/anti-counterfeiting/index.php.
- DuPont Izon. DuPont. Accessed at www2.dupont.com/Authentication/en_US/products_services/ izon_3d_holographics.html.
- Countering the Problem of Falsified and Substandard Drugs. Institute of Medicine of the National Academies, February 2013. Accessed at www.iom.edu/Reports/2013/Counteringthe-Problem-of-Falsified-and-Substandard-Drugs.aspx.
- Wikipedia. Epedigree. Accessed at en.wikipedia.org/wiki/Epedigree.
- U.S. Food and Drug Administration. Counterfeit Medicine. Accessed at www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/CounterfeitMedicine/default.htm.
- New Counterfeit Avastin Found. The Partnership for Safe Medicines. Accessed at www.safemedicines.org/2013/02/new-counterfeit-avastin-found-medical-practitionersadvised-by-fda-to-be-wary-of-unfamiliar-wholesal-513.html.
- RxAssist. Accessed at www.rxassist.org.
- Counterfeit Drugs. National Association of Boards of Pharmacy. Accessed at www.nabp.net/programs/consumer-protection/buying-medicine-online/counterfeit-drugs.
- U.S. Food and Drug Administration. Reporting of Counterfeit Drug Products. Accessed at www.fda.gov/Drugs/DrugSafety/ucm170314.htm.