340B Drug Discount Program in 2017
- By BSTQ Staff
The 340B Drug Discount Program is a price control program for drugs purchased by nonmilitary and VA healthcare entities overseen by the Health Resources and Services Administration (HRSA) within the Department of Health and Human Services. Briefly, it requires participating drug manufacturers to give certain entities within the healthcare safety net, known as covered entities (see Eligible Entities), access to discounted prices on outpatient drugs. The program’s intent is to allow these entities to increase patient services with savings they accrue by purchasing product at a lower cost.
The program started in 1990 when Congress created the Medicaid rebate program to lower the cost of covered outpatient drugs reimbursed by state agencies. Relief from high drug costs for safety-net providers (those that care for people without health insurance, who are poor or who may be on Medicaid) was extended in 1992 when Congress enacted Section 340B of the Public Health Services Act, created under the Veterans Health Care Act of 1992.
The three components of the program include the Office of Pharmacy Affairs (OPA), Pharmacy Services Support Center and 340B Prime Vendor Program. To participate, covered entities must register with HRSA, and if approved, they are added to the approved entities database. Eligibility is redetermined annually. Once approved, they may purchase covered outpatient drugs under the 340B program at or below the 340B ceiling price.
Manufacturers are obligated to calculate 340B prices accurately using a statutorily defined formula and to communicate prices to distributors. At a minimum, the discount is 23.1 percent for brand-name drugs (except clotting factor and drugs approved exclusively for pediatric use, for which the basic rebate is 17.1 percent of average manufacturer price) and 13 percent for generic and over-the-counter drugs. Additional discounts can be negotiated, some up to 60 percent below retail prices and 51 percent less than average wholesale price. With adequate inventory, manufacturers must sell product to enrolled covered entities and cannot condition sales on a promise of compliance. Manufacturers retain the right to audit a covered entity if reasonable evidence suggests noncompliance with prohibition against duplicate discounts or diversion.
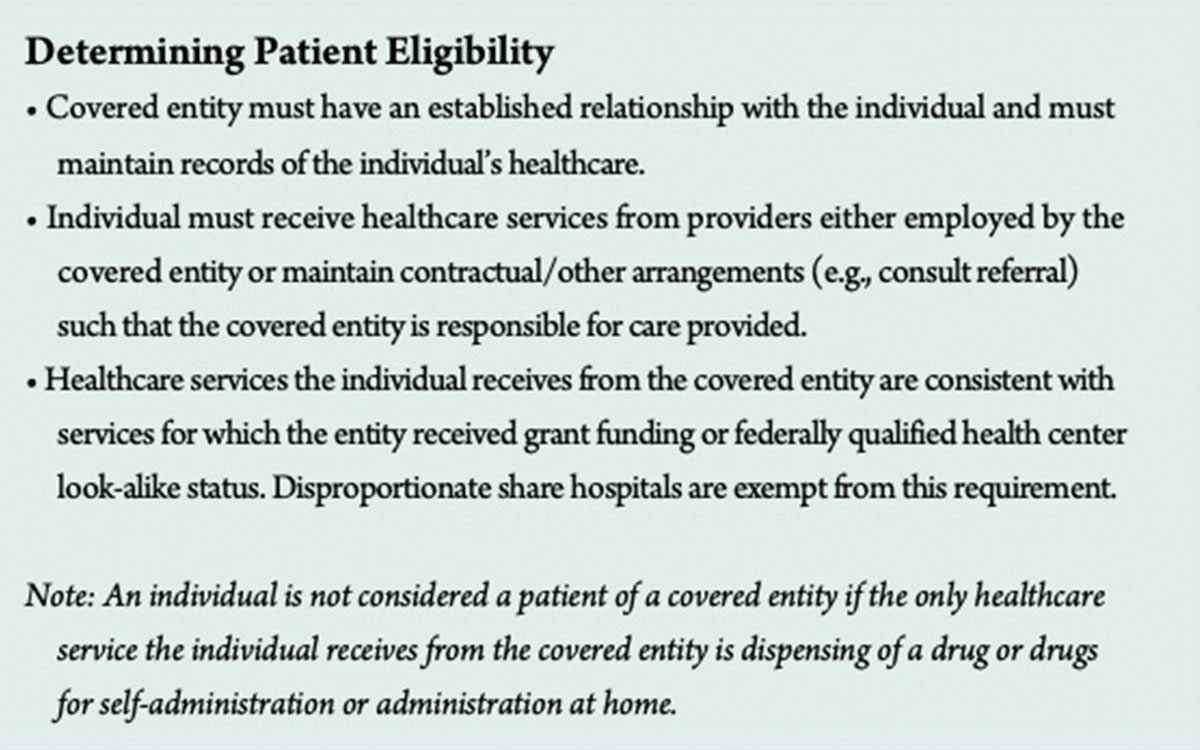
Program prohibitions include diversion of drugs to ineligible patients (see Determining Patient Eligibility) and duplicate discounts that occur when a facility is obtaining the 340B discount plus the Medicaid rebate on the same drug. Subjecting drug manufacturers to duplicate discounts on 340B-purchased drugs is prohibited by law, so covered entities are required to report their Medicaid billing status. They can choose to dispense 340B-purchased drugs to Medicaid beneficiaries, and they can indicate their choice in HRSA’s covered entity database, which state Medicaid agencies use to identify Medicaid payments for 340B-purchased drugs that are excluded from rebate requests to drug manufacturers.

340B Oversight: Not What It Used to Be
Due to the increase in number of users of the 340B program by the Affordable Care Act’s expansion of it and HRSA’s allowance to contract with multiple pharmacies, significant changes in oversight were required, including annual enrollment recertification, audits of 340B covered entities and manufacturer audits.
On Aug. 25, 2015, HRSA released the proposed 340B “mega-guidance” that addressed program eligibility and registration, eligibility of drugs for purchase under 340B, patient eligibility to receive 340B drugs, requirements for covered entities, arrangements for contract pharmacies, manufacturer responsibilities, rebate options for AIDS drug assistance programs and program integrity. But, after multiple comments were received and addressed, on Jan. 30, the White House Office of Management and Budget (OMB) withdrew the final guidance document. It is unknown as of this writing whether HRSA’s guidance will move forward with resubmission to the OMB.
What is known is that 340B participants must be confident about the integrity of their 340B program. Myriad details need to be reviewed, including business processes, policies and procedures; enrollment and vendor contracts; diversion of 340B drugs to ineligible patients; duplicate discounting; the group purchasing organization exclusion requirement; identification and definition of all eligible patients and drugs; issues with accumulator/splitter software; oversight of contract pharmacy activity, inventory balances and contract compliance; duplicate dispenses; replenishment of 340B drugs; orphan drugs; monitoring and reporting of 340B transactions; and identification of missed opportunities to receive 340B discounts.
Why the Sudden and Expanded Interest in 340B?
Currently, there is uncertainty about the program, with conflicting viewpoints by opponents (stakeholders and regulatory bodies) and participating supporters, as well as non-340B facilities clamoring to be a part of it due to the astronomical rise in drug prices, especially in the oncology and immunotherapy areas.
However, HRSA is faced with many challenges. To be poised for growth, it must address insufficient capacity, technology restructure and bolstered resources. Certainly, the furor over drug pricing highlighted by congressional hearings is keeping the program in the limelight. Additionally, the January 2016 MedPAC recommendation to Congress to cut Medicare payments to safety net hospitals by 10 percent for drugs purchased under the 340B drug program may still be viable. The Centers for Medicare and Medicaid covered outpatient drug rule and the Medicaid rebate rule may be addressed as well.
Limited distribution drugs, specialty drugs and orphan drugs (some of the most expensive products) are hot-button issues in the 340B program. Orphan drugs, which are excluded from 340B pricing for some participants, are worthy of particular attention since the U.S. Food and Drug Administration continues to receive a record number of requests for orphan drug designation. While drugs can have multiple indications, they qualify for orphan drug designation only when used to treat certain rare diseases or conditions. The rationale for the orphan drug program is to incentivize manufacturers to develop drugs for rare conditions. Designated drugs maintain that status for an indefinite period.
The HRSA website lists more than 3,400 drugs with orphan drug status. Rural referral centers, sole community hospitals, critical access hospitals and free-standing cancer hospitals participating in the 340B program are vulnerable since manufacturers are not required to provide them with orphan drugs under the 340B program. However, manufacturers may, at their sole discretion, offer discounts on orphan drugs to them (see docs.340bpvp com/documents/public/resourcecenter/Summary_Orphan_Drugs.pdf).
A Time to Prepare
Although the mega-guidance has been stalled, facilities can still prepare by performing audits to manage and confirm compliance with current 340B program requirements, and evaluating the financial and operational effects of any proposed changes. And, because there is no presumptive eligibility in the 340B program, facilities can ensure best practices are in place by:
- Developing and documenting comprehensive 340B policies and procedures;
- Developing concrete methodologies for routine self-monitoring;
- Implementing routine processes for internal corrective action;
- Verifying contract pharmacy arrangements comply with 340B requirements and are properly listed in the OPA database; and
- Creating strong partnerships with state Medicaid agencies to meet state-specific requirements and to ensure prevention of duplicate discounts.