Choosing Influenza Vaccines
With the advent of new products with new manufacturing processes and added protection, the number of available influenza vaccine presentations has risen to 13. Which ones to choose for your patients can be simplified with a look at the pros and cons of the new compared with the traditional.
- By Ronale Tucker Rhodes, MS
Choosing which influenza vaccines to administer to your patients these days is more complicated than it used to be. In just the past year, five new vaccines have been approved by the U.S. Food and Drug Administration (FDA), and there are reports of even more coming to market as we begin the 2013-2014 flu season. Instead of replacing the current ones, almost all of these vaccines are just being added to the list, making purchasing decisions more complex.
Manufacturers are on a quest to make vaccines that will provide more protection, but in doing so, both patients and healthcare providers are confused about which ones best suit their needs. For instance, what are the advantages of an egg-based or cell-culture vaccine? Do four strains in the quadrivalent inactivated vaccines (IIV4s) really provide that much more protection than the three strains included in the trivalent inactivated vaccines (IIV3s)? What about whether the vaccines and/or vaccine packaging contain additives? And, let’s not forget that the new vaccines cost more. Are the added protections in the new vaccines really worth that additional cost?
What Vaccines Have Been Available
Prior to this year, nine influenza vaccines have been available, including FluLaval and Fluarix (GlaxoSmithKline), Afluria (Merck/CSL), Fluvirin and Agriflu (Novartis Vaccines), Fluzone, Fluzone Intradermal and Fluzone High-Dose (Sanofi Pasteur) and FluMist (MedImmune). All of these vaccines are IIV3, with the exception of FluMist, a live-attenuated influenza vaccine (LAIV4). It is the only one that has been completely replaced by a new quadrivalent formulation for the 2013-2014 flu season. All of these vaccines are produced annually according to the formulation identified by the FDA and the World Health Organization (WHO) that contains the isolated influenza virus strains thought most likely to be circulating and causing illness among people during the upcoming flu season. The differences among these vaccines range from how they are manufactured, how they are packaged, the age groups for which they are indicated, the number of doses required, the route of administration, and whether the vaccines contain preservatives or other additives. See Table 1 for specifications for each of these vaccines.
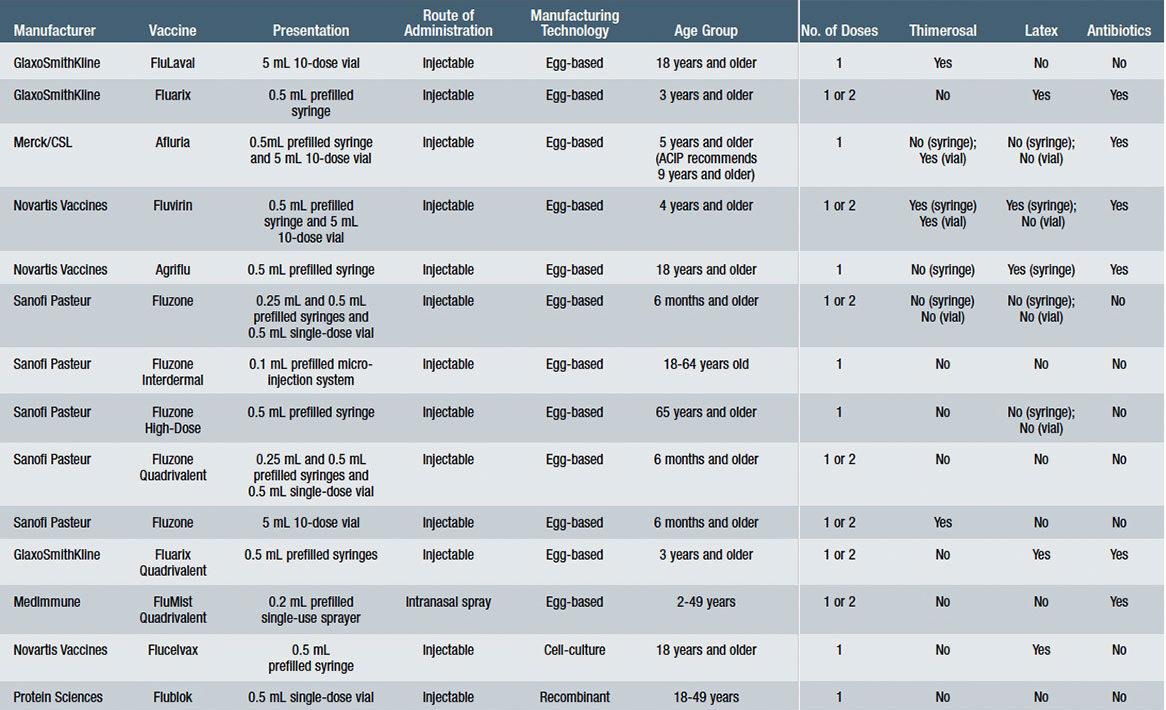
The Challenges of Influenza Vaccine Production
For years, the most troublesome issues surrounding influenza vaccines include the manufacturing process and the identified vaccine strains’ potential protective benefit. To address these issues, many manufacturers have been researching ways of producing flu vaccines using new methods, and the FDA and WHO have continued to struggle to predict the correct virus strains to include in the vaccines, achieving a match with the circulating strains just five out of 10 years during the decade beginning with the 2001-2002 flu season.
Furthermore, there are several problems with the 60-year-old production method that requires the strains of the influenza vaccines be grown in fertilized chicken eggs, which can take up to six months. In the event of a pandemic, such as the potential one that occurred in 2003 with the re-emergence of the H5N1 bird flu and the actual pandemic that occurred in 2009 with the H1N1 swine flu, the egg-based production method cannot supply enough influenza vaccine to adequately protect the public. It became especially worrisome that the U.S. would have a sufficient supply when in 2004, the U.S. flu vaccine supplies were devastated by contamination at a plant in Liverpool, England, underscoring the need for the U.S. to have its own manufacturing capabilities, says Robin Robinson, director of the U.S. Biomedical Advanced Research and Development Authority (BARDA), a part of the U.S. Department of Health and Human Services (HHS). Should a pandemic occur, the fear was that other countries might be tempted to commandeer all flu vaccines made within their own borders, leaving the U.S. without enough vaccines. “We needed to develop new vaccines using modern technologies that would make not only more vaccine available sooner, but also make it more effective,” explains Robinson.1 Added to these issues are the inherent problems with the manufacturing process, plus egg-related complications. At every step of the process, there is risk of contamination, and in some years, certain flu strains have refused to grow readily in eggs. And, people with severe allergic reaction (anaphylaxis) to eggs can’t get a flu shot. Notably, eggs happen to be one of the most common food allergens; in the U.S., more than 600,000 people have egg allergies.2
To combat these issues, in 2006, the HHS provided more than $1 billion in contracts to six manufacturers to develop cell-culture-based flu vaccine technology in the U.S. Then, in 2009, when it was difficult to grow vaccine to respond to the H1N1 swine flu pandemic, the HHS granted Novartis nearly $500 million to build the first U.S. facility capable of producing cell-based vaccine for seasonal and pandemic flu in the U.S. (Novartis footed the additional $1 billion price tag.) Also in 2009, the HHS awarded a five-year, $147 million investment to Protein Sciences, which was investigating a recombinant vaccine that is grown inside insect cells.1 With cell-culture-based and recombinant production techniques, influenza vaccines can be produced easier and faster — within weeks — for seasonal or pandemic influenza. And, because the vaccine is grown in sterile, controlled environments, the risk of potential impurities is significantly reduced.3
The other most troublesome issue concerns the vaccines’ effectiveness. In some years, influenza vaccines protect only 50 percent to 70 percent of people who receive them. According to the Centers for Disease Control and Prevention’s mid-season vaccine effectiveness (VE) estimates published on Feb. 21, 2013, the 2012-2013 VE for protecting against having to go to the doctor because of flu illness was 56 percent for all age groups. When broken down by age groups, the VE against flu A and B viruses ranged from 27 percent in people 65 and older, to 64 percent in children aged 6 months to 17 years.
Predicting which strains of the virus to include in the influenza vaccines is difficult at best, not only because the virus mutates from year to year but the number of influenza subtypes A and type B that can be selected for inclusion is limited. IIV3s help protect against the two A virus strains most common in humans and the B strain expected to be predominant in a given year. But, since the year 2000, two influenza B lineages (Victoria and Yamagata) have co-circulated to varying degrees each season. Various degrees of mismatch have occurred between the B lineage included in IIV3s and the B lineage that actually circulated, causing an increased risk of influenza-related morbidity across all age groups. “Trivalent influenza vaccines have helped protect millions of people against flu, but in six of the last 11 flu seasons, the predominant circulating influenza B strain was not the strain that public health authorities selected,” says Dr. Leonard Friedland, vice president and head of GlaxoSmithKline North America Vaccines Clinical Development and Medical Affairs.4
Adding a second B strain to the seasonal vaccine had been discussed for years. The problem with doing this, however, was the lack of adequate manufacturing capacity to produce IIV4s that still allowed manufacturers to make enough doses to meet projected demand. “From the 2001-2002 through the 2005-2006 flu seasons, fewer than 100 million doses of seasonal flu vaccine were produced and distributed in the U.S.,” says Keith Berman, founder of Health Research Associates. “But since 2005-2006, flu vaccine manufacturing capacity has dramatically expanded — a direct byproduct of avian and swine flu outbreaks that prompted the U.S. government to help industry improve preparedness for a potential global flu pandemic.” Over the last two flu seasons, manufactured doses of influenza vaccines have outpaced market demand, and “for the first time, the vaccines industry finds itself with the capacity to inoculate many millions more eggs to produce large stocks of IIV4 without jeopardizing its ability to make enough doses to satisfy market demand,” adds Berman.5
The benefit of adding a second B lineage to influenza vaccines is “essentially a matter of chance,” says Berman. However, as an example of how it could make a difference, in the 2007-2008 flu season, B viruses accounted for 29 percent of all flu infections. Unfortunately, WHO and FDA virologists picked the wrong B lineage: the Victoria lineage vs. the Yamagata lineage. Had they added the Yamagata lineage that was identified in 98 percent of the flu cases with a B virus infection, the CDC estimates that nearly one million flu illnesses and 484 deaths could have been averted. The next year also serves as an example. In the 2008-2009 flu season, officials picked the wrong lineage again. Had both B lineages been included in the influenza vaccines, the CDC estimates that 169 lives could have been saved.5
Widespread avoidance of the influenza vaccination remains yet another issue. On average, the number of people who get a flu shot each year hovers in the 40 percent range.6 The reasons vary, but mainly it’s due to misconceptions that the flu shot causes the flu, that the flu shot causes unwanted side effects, that it doesn’t work and, for many, it’s a fear of needles. While the first three reasons are known to be myths, a fear of needles is all too real. Which is why the HHS is now focusing on a universal vaccine that could be given every five to 10 years, much like a tetanus shot. The universal vaccine also would protect against most types of flu, including seasonal varieties and the highly mutated kinds that cause pandemics.1
A final issue surrounding influenza vaccines are additives that are introduced into the vaccines through the manufacturing process. These additives include thimerosal, antibiotics and latex — all of which may cause problems in individuals with allergies to them. Thimerosal is a mercury-containing organic compound that has been widely used since the 1930s as a preservative in vaccines to help prevent potentially life-threatening contamination with harmful microbes. Because public concerns about the use of thimerosal in vaccines and other products have been raised, the FDA is working with manufacturers to reduce or eliminate thimerosal from vaccines. Most influenza vaccines have very low, trace or no thimerosal levels.7
Certain antibiotics, including neomycin, polymyxin B, streptomycin and gentamicin, also may be used in making inactivated influenza virus vaccines to help prevent bacterial contamination during manufacturing. Antibiotics used in vaccine production are present in some vaccines, but they are reduced to very small or undetectable amounts during subsequent purification steps. And, the very small amounts of antibiotics contained in vaccines have not been clearly associated with severe allergic reactions.8
Some influenza vaccine packaging, including syringes, plungers and vial stoppers, may contain latex, to which some people are allergic. According to the 2011 general recommendations on immunization by the Advisory Committee on Immunization Practices: “If a person reports a severe (anaphylactic) allergy to latex, vaccines supplied in vials or syringes that contain natural rubber should not be administered unless the benefit of vaccination outweighs the risk for a potential allergic reaction. In these cases, providers should be prepared to treat patients who are having an allergic reaction. For latex allergies other than anaphylactic allergies (e.g., a history of contact allergy to latex gloves), vaccines supplied in vials or syringes that contain dry natural rubber or rubber latex may be administered.”9
What’s New in Influenza Vaccines
As a result of the HHS response to the two most troublesome issues surrounding influenza vaccines, five new vaccines have entered the market for the 2013-2014 flu season, more are planned for this season, and even more are on the horizon.
In February 2012, the FDA approved the first LAIV4, FluMist Quadrivalent, manufactured by MedImmune. The vaccine is approved for individuals aged 2 years through 49 years, and it contains four strains of the influenza virus: two A strains and two B strains. Like the LAIV FluMist (which has been removed from the market for the new flu season), the LAIV4 contains weakened forms of the virus strains and is administered as a nasal spray. The safety and effectiveness of FluMist Quadrivalent is supported by studies conducted previously for the LAIV FluMist, as well as three new clinical studies conducted in the U.S. involving 4,000 children and adults, that demonstrated that the immune responses were similar between FluMist and FluMist Quadrivalent. Reported adverse reactions also were similar, including runny or stuffy nose in both children and adults and headache and sore throat in adults.10
Then, in December 2012, a second IIV4 was approved by the FDA. Fluarix Quadrivalent, manufactured by GlaxoSmithKline, is the first intramuscular vaccine to protect against four influenza strains, and it is approved for individuals aged 3 years and older. In clinical trials, the most common adverse reactions in adults were pain at the injection site, muscle aches, headache and fatigue. In children between 3 years and less than 6 years, the most common adverse reactions were drowsiness, irritability and loss of appetite. And, in children 6 years to less than 18 years, the most common systemic adverse reactions were fatigue, muscle aches, headache, arthralgia and gastrointestinal symptoms.11
Last month, the FDA approved Sanofi Pasteur’s Fluzone Quadrivalent for use in children 6 months and older, adolescents and adults. It is the first IIV4 option for children as young as 6 months. The vaccine comes in preservative-free, prefilled syringes and single-dose vials for intramuscular administration. In clinical trials, the most common local and systemic adverse reactions were pain, erythema and swelling at the vaccination site; myalgia; malaise; headache; and fever. In some young children, the vaccine also caused irritability, crying and drowsiness.
In January, the FDA approved the first two new influenza vaccines using non-egg-based technologies, making flu vaccines available to the hundreds of thousands of individuals allergic to eggs, as well as providing an easier methodology of producing influenza vaccines at a faster rate both for seasonal influenza and in the event of a flu pandemic.
Novartis’ Flucelvax is manufactured using MDCK cell-culture technology, and it is approved for individuals 18 years and older. The ccIIV3 vaccine is produced through four steps. First, the seed stocks for three influenza viruses are produced. Then, the virus is propagated in cells that are expanded and inoculated with the influenza viruses and allowed to replicate over several days. The virus is then isolated, inactivated and purified by removing the influenza-antigen components. Finally, the virus is formulated by combining the antigen components into one vaccine. In seven controlled studies of Flucelvax, the rates of serious adverse events were collected for 21 days in two studies and for six to nine months in five studies. Subjects were divided into three groups, one that received Flucelvax, the other that received a U.S.-licensed comparator vaccine and a third that received a placebo. In each of these groups, the rate of all serious adverse events among adults 18 through 64 years of age was 1 percent. The rate of serious adverse events among adults 65 years of age and older was 4 percent in both groups that received Flucelvax and those that received a U.S.-licensed comparator vaccine. Flucelvax contains no additives or preservatives.12
Protein Science’s Flublok is manufactured using an insect virus (baculovirus) expression system and recombinant DNA technology. The recombinant production process involves programming insect cells grown in steel tanks to produce large amounts of a particular protein, known as hemagglutinin. The majority of antibodies that prevent influenza virus infection are directed against hemagglutinin. The RIV3 is designed to protect against the H1N1, H3N2, both A strains and one B strain of the influenza virus, and it is approved for people between the ages of 18 and 49. In a study of 2,300 people, the vaccine was found to be 44.6 percent effective against all strains of the flu. Flublok’s safety evaluation was conducted in a study of about 2,500 people who were vaccinated with Flublok. The most common side effects included muscle aches, headache, fatigue and pain in the area the shot was administered. This vaccine also contains no additives or preservatives.13
While both the cell-culture-based and recombinant technologies are new to flu vaccine production, they are used to make vaccines that have been approved by the FDA to prevent other infectious diseases. See Table 1 for specifications for each of these vaccines, as well as the quadrivalent vaccines.
These five new vaccines are not the only result of HHS efforts. Novartis Vaccines also is developing egg-based and cell-culture-based quadrivalent products.5 And, two other genetically engineered flu vaccines also are under development. One by Novavax uses bits of genetic material grown in caterpillar cells called “virus-like particles” that mimic a flu virus. The other is being developed by VaxInnate Corp. In 2011, the HHS awarded VaxInnate a five-year, $196 million grant to make a vaccine that combines a bacterial protein called flagellin, a potent stimulator of the immune system, with a very small portion of hemagglutinin. VaxInnate’s flu vaccine is in mid-stage clinical trials. Both of these vaccines are expected to be available in the latter part of the decade.1
It’s not known how soon a universal influenza vaccine could be made available. However, while several teams have tried and failed to produce such a vaccine, scientists at the National Institute of Allergy and Infectious Disease (NIAID), a part of the National Institutes of Health (NIH), and others are making good progress, according to Dr. Anthony Fauci, director of the NIAID. Dr. Fauci and Dr. Gary Nabel, former head of NIH’s Vaccine Research Center who recently joined Sanofi Pasteur as chief science officer, showed that a portion of the flu virus that is usually hidden from the immune system may be the key. Most vaccines target proteins on the bulb portion of the hemagglutinin part of the flu virus, which mutates from year to year. But, the stem portion, which contains proteins that are structurally hidden from the immune system, don’t change much from virus to virus. A genetically engineered vaccine could overcome that by presenting only the stem proteins to the immune system. Phase I studies have begun in people to test for safety and whether the vaccine can create an appropriate immune response. Novartis Vaccines and BARDA will be handling the manufacturing of the vaccine.1
Weighing the Cost vs. the Benefit
The new cell-culture-based vaccines and quadrivalents do come with an additional cost, and many question whether the added expense is worth choosing these vaccines for patients over the less-expensive ones that are on the market. The answer to that question lies in the safety and effectiveness of the new vaccines. Of course, as recent entries on the market, no data from annual influenza infection rates are available yet to prove their safety and efficacy. And, it is possible that not everyone needs to be inoculated with the new vaccines. But those who have allergies to eggs now can receive a flu shot, potentially adding 600,000 to the ranks of people protected from the influenza virus. And, considering the challenge of choosing the correct B lineage to include in the trivalent seasonal influenza vaccine, the chances of individuals gaining more protection from including both B lineages in the quadrivalent cannot be argued. This extra protective edge could be especially important for high-risk populations most susceptible to succumbing from influenza.
Added protection also can positively influence the high cost to society caused by influenza. A recent study examined the additional influenza cases that a quadrivalent may have averted during the past decade (influenza seasons 1999 through 2009) to determine the potential cost-savings a quadrivalent may provide. The researchers divided influenza cases into three categories: those who were infected without requiring hospitalization, those who required hospitalization and survived, and those who were hospitalized and died. They also divided the quadrivalent into different price premiums of $5, $15, $30 and $120 more than a trivalent. These translated to a median of $3.1 billion societal cost savings and a median of $292 million third-party payer cost savings during the decade if the quadrivalent were used instead of the trivalent and priced equally to the trivalent. Over the decade, 2,684,145 total cases were averted with a quadrivalent vaccine. From the third-party payer perspective, a $120 premium would have saved $11 per case and a $0 premium would have saved $109 per case across the decade. Cost savings per case across the decade from the societal perspective ranged from $1,163 ($0 premium) to $1,041 ($120 premium). The cost per case tended to increase as premiums decreased, resulting in less cost savings. The researchers concluded that “adding an additional B strain to the seasonal influenza vaccine could reap substantial cost savings for society and third-party payers, even if the quadrivalent enjoyed a significant price premium over the trivalent.”14
Currently, less than half of the U.S. population gets a flu shot each year, despite the grim statistics that influenza affects from 5 percent to 20 percent of the population, claiming a range of 3,000 to 49,000 lives and requiring hospitalization of more than 200,000 suffering from influenza-associated illnesses. Without at least 90 percent of the population becoming vaccinated, herd immunity, which provides sufficient protection to stop the spread of disease, cannot be achieved. If the goal is to increase the numbers of individuals vaccinated against the often-deadly influenza virus, perhaps the greatest hope to boost vaccination rates lies with the improvements offered by the new vaccines.
References
- Steenhuysen J. Insight: U.S. Government Investment Gives Flu Vaccines a Shot in the Arm. Reuters, Jan. 19, 2013. Accessed at www.reuters.com/article/2013/01/19/us-flu-vaccinesidUSBRE90I0AI20130119.
- American College of Allergy, Asthma & Immunology. Egg Allergy. Accessed at www.acaai.org/allergist/allergies/Types/food-allergies/types/Pages/egg-allergy.aspx.
- American Pharmacists’ Association. Flucelvax: First Seasonal Vaccine Using Cell-Culture Technology. Jan. 1, 2013. Accessed at www.pharmacist.com/flucelvax-first-seasonalvaccine-using-cell-culture-technology.
- GlaxoSmithKline. FDA Approves GlaxoSmithKline’s Four-Strain Seasonal Influenza Vaccine for Use in the U.S. Dec. 17, 2012. Accessed at www.gsk.com/media/pressreleases/2012/FDA-pproves-GlaxoSmithKline-four-strain-seasonal-influenza-vaccine-foruse-in-the-US.html.
- Berman K. Quadrivalent Flu Vaccines: Four Means More Protection. BioSupply Trends Quarterly, July 2012. Accessed at www.bstquarterly.com/archived_issues.aspx.
- Centers for Disease Control and Prevention National Center for Immunization and Respiratory Disease Immunization Services Division. Influenza Vaccination Distribution and Coverage, United States, 2010-11 and 2011-12 Seasons. Oct. 26, 2011. Accessed at www.cdc.gov/flu/fluvaxview/trends.htm.
- U.S. Food and Drug Administration. Vaccines, Blood & Biologics: Thimerosal in Vaccines. Accessed at www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228.
- U.S. Food and Drug Administration. Common Ingredients in U.S. Licensed Vaccines. Jul. 7, 2011. Accessed at www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/ucm187810.htm.
- Centers for Disease Control and Prevention. Latex in Vaccine Packaging. Accessed at www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/latex-table.pdf.
- U.S. Food and Drug Administration. FDA Approves First Quadrivalent Vaccine to Prevent Seasonal Influenza. Accessed at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm294057.htm.
- GlaxoSmithKline. FDA Approves GlaxoSmithKline’s Four-Strain Seasonal Influenza Vaccine for Use in the U.S. Accessed at us.gsk.com/html/media-news/pressreleases/2012/2012-pressrelease-1279670.htm.
- Novartis. Flucelvax (Influenza Virus Vaccine) Fact Sheet. Accessed at www.novartisvaccines.com/…/flucelvax/Flucelvax_Fact_Sheet.pdf.
- U.S. Food and Drug Administration. FDA Approves New Seasonal Influenza Vaccine Made Using Novel Technology. Accessed at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm335891.htm.
- Lee BY, Bartsch SM, and Willig AM. The Economic Value of a Quadrivalent Versus Trivalent Influenza Vaccine. Vaccine 30 (2012); 7443-7446.