Long-Acting Factor Concentrates: The Next Leap Forward in Hemophilia Care Arrives
- By Keith Berman, MPH, MBA
Innovation is the calling card of the future. — Anna Eshoo
FEW IN NUMBER are successful collaborations between clinical scientists and industry that eventually transform a generally devastating or seriously debilitating chronic disease into a readily manageable condition compatible with a long and healthy life.
One such disorder is type 1 diabetes, which once committed most young victims to a life diminished by cardiovascular disease, retinopathy, neuropathy and nephropathy — the most serious results of chronic poorly controlled blood glucose. Numerous innovations relating to the form and delivery of insulin — multiple daily insulin injection (MDII), insulin products with varying onsets of action, continuous glucose monitoring and insulin pump therapy — have dramatically improved glucose control and long-term prognosis.
A succession of treatment advances over the last 40 years has similarly transformed the lives of children and adults with hemophilia. The transition in the early 1970s from transfused fresh frozen plasma or cryoprecipitate to self-administered plasma-sourced factor VIII (hemophilia A) and IX (hemophilia B) concentrates immediately translated into less severe bleeds and reduced long-term joint disease. Later development of validated pathogen separation and inactivation processes and recombinant human factor VIII and IX products have virtually eliminated HIV and hepatitis infection risk.
Beginning in the 1990s, prescription of long-term prophylaxis regimens to maintain the circulating factor VIII level above 1 percent to 3 percent of normal (and factor IX typically above 2 percent of normal) has helped patients with more severe disease sharply cut the number of breakthrough bleeding episodes. Under the care of a well-trained hemophilia treatment specialist, an infant male born today with a severe factor VIII deficiency can prophylactically self-administer factor VIII every other day to three times weekly* to minimize or avert serious bleeding events and irreversible joint damage, and live a long, active life.
But not unlike type 1 diabetics who maintain glycemic control with MDII therapy, a price must be paid by persons with hemophilia who commit to routine prophylaxis: the need for frequent self-injections. The diabetes management industry responded to needlestick-related treatment compliance problems and other shortcomings of MDII by developing continuous subcutaneous insulin infusion (CSII) by self-programmable insulin pumps and, very recently, the creation of a new inhalable insulin product used in conjunction with long-acting insulin.1 But for persons with hemophilia on a prophylaxis regimen, there is no option other than frequent intravenous (IV) self-administration of their factor product.
Today nearly two-thirds of persons with moderate or severe forms of hemophilia A, and one-third with hemophilia B, manage their disease with some form of prophylaxis.2 But, unfortunately, some fail to reliably adhere to their prescribed schedule of regular infusions of clotting factor. Poor compliance results in periods of sub-protective levels of circulating factor VIII or IX and consequent breakthrough bleeding events. But even for those who have the discipline to do so, self-administration of their drug into a vein several times a week is an unpleasant and burdensome experience.
Of course the optimal solution is to cure hemophilia itself through gene therapy. A number of clinical and preclinical studies are currently in progress to investigate various gene therapy candidates, but prospects for approved treatments remain uncertain. For hemophilia A in particular, which accounts for about 80 percent of all persons with moderate and severe disease, safe and effective gene therapy remains many years away.
Meanwhile, the biopharmaceutical industry has pursued the next best option: Reduce the number of necessary infusions. As with other long-acting versions of approved biotherapeutics, this is achievable by variously modifying factor VIII or IX to persist longer in the circulation while still retaining the protein’s key functionality in the blood coagulation pathway. The need, as well as the commercial stakes involved, are well-appreciated: Five leading biopharmaceutical firms have invested heavily in development of eight proprietary long-acting factor VIII and IX product candidates.
First Long-Acting Hemophilia Therapies Have Arrived
In the race to introduce these products are most of the major suppliers of today’s conventional recombinant coagulation factors: Baxter, Bayer, CSL Behring and Novo Nordisk. But the first to secure regulatory approvals for its both long-acting factor VIII and factor IX products — Biogen Idec — turns out to be an entirely new entrant to the hemophilia therapy market. In March, the U.S. Food and Drug Administration (FDA) approved its ALPROLIX (Factor IX [Recombinant], Fc Fusion Protein), followed just three months later by approval of ELOCTATE (Antihemophilic Factor [Recombinant], Fc Fusion Protein).
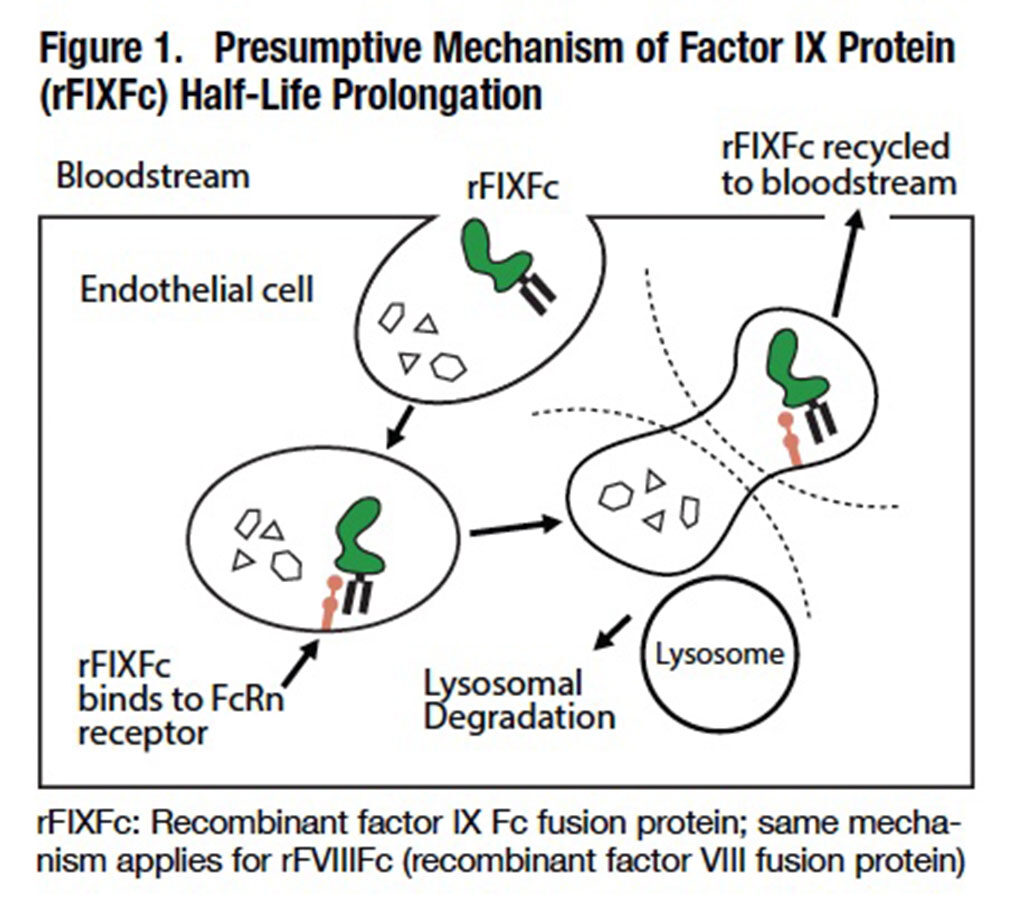
Fusing the Fc portion of human IgG1 is believed to extend the half-life of these recombinant clotting proteins by exploiting the natural process that enables IgG to persist in the circulation for several weeks. Following endocytosis by vascular endothelial cells, the factor protein-bound constant Fc region of IgG1 binds to cellular neonatal Fc receptors (FcRn), which divert the protein-Fc complex away from lysosomes and facilitate its return to the bloodstream (Figure 1). The half-life of Biogen Idec’s ALPROLIX factor IX fusion protein is about 86 hours, compared with about 26 hours for Baxter’s RIXUBIS and just under 19 hours for Pfizer’s BeneFIX recombinant factor IX products. The half-life of ELOCTATE, a much larger factor VIII fusion protein, is increased about 1.5-fold over the mean half-life of 12 hours for adults treated with Baxter’s ADVATE, the market-leading conventional recombinant factor VIII.
This Fc fusion technology is hardly new: The first of seven Fc fusion-based protein therapeutics approved in the U.S. over the last 15 years was the anti-tumor necrosis factor drug Enbrel (etanercept) in 1998, whose worldwide sales for treatment of autoimmune rheumatologic disorders now reportedly top $8 billion. Antibody-based therapeutics that utilize IgG1 Fc fusion to prolong half-life now account for about 20 percent of all licensed antibody-based medicines.3
Whether long-acting or not, the therapeutic principle behind factor VIII or IX prophylaxis is the same: Keep the trough level of the missing clotting factor above a low threshold associated with increased bleeding risk. Pharmacokinetic simulations of Biogen Idec’s long-acting factor VIII found a clear association between number of days with a factor VIII activity under 1 international unit (IU)/dL and increased bleeding tendency. A mean of 224 out of 365 days under 1 IU/dL was documented in clinical trial subjects receiving solely on-demand treatment with ELOCTATE. The annualized period under 1 IU/kg was shortened to about 52 days in subjects managed with a fixed prophylactic dose of 65 IU/kg once weekly, and further shortened to just two days in subjects on individually tailored prophylactic regimens.
Thus, for each patient managed prophylactically with ELOCTATE or any factor concentrate, a balance must be found between increasing the time interval between infusions and maintenance of a protective clotting factor activity level. In the pivotal ELOCTATE clinical study, pharmacokinetic parameters were used to guide the dosing interval (every three to five days) and dose (25 to 65 IU/kg) in a group of subjects on individualized prophylaxis; the mean annualized bleeding rate (ABR) in this group was 95 percent lower than the ABR in the on-demand group. The ABR in a separate group managed with weekly prophylaxis at a fixed 65 IU/kg dose was 90 percent lower than the on-demand group — good, but still a meaningfully higher rate than the group on individualized prophylaxis. Consistent with these findings, the prescribing information for ELOCTATE advises physicians to start with 50 IU/kg every four days, then individualize dose and injection frequency based on patient response.
The practical advantage of ELOCTATE for patients is straightforward: A prophylaxis regimen can maintain a protective factor VIII level with fewer self-infusions than conventional recombinant factor VIII products while realizing similar benefits in reduced breakthrough bleeds compared with on-demand treatment. An individual who required conventional factor VIII dosing three times weekly, for example, may need to dose ELOCTATE only twice a week. Similarly, because its elimination half-life is several times longer than that of Pfizer’s BeneFIX, Baxter’s RIXUBIS or plasma-based factor IX products, including AlphaNine SD and Mononine, the frequency of ALPROLIX prophylaxis can be significantly reduced.
Other Long-Acting Factors Advance Toward Approval
Biogen Idec currently offers the only licensed long-acting factor VIII and IX products, but that advantage is likely to be short-lived. Four investigational recombinant factor VIII and two recombinant factor IX products with extended half-lives are now in advanced clinical development (Tables 1 and 2).
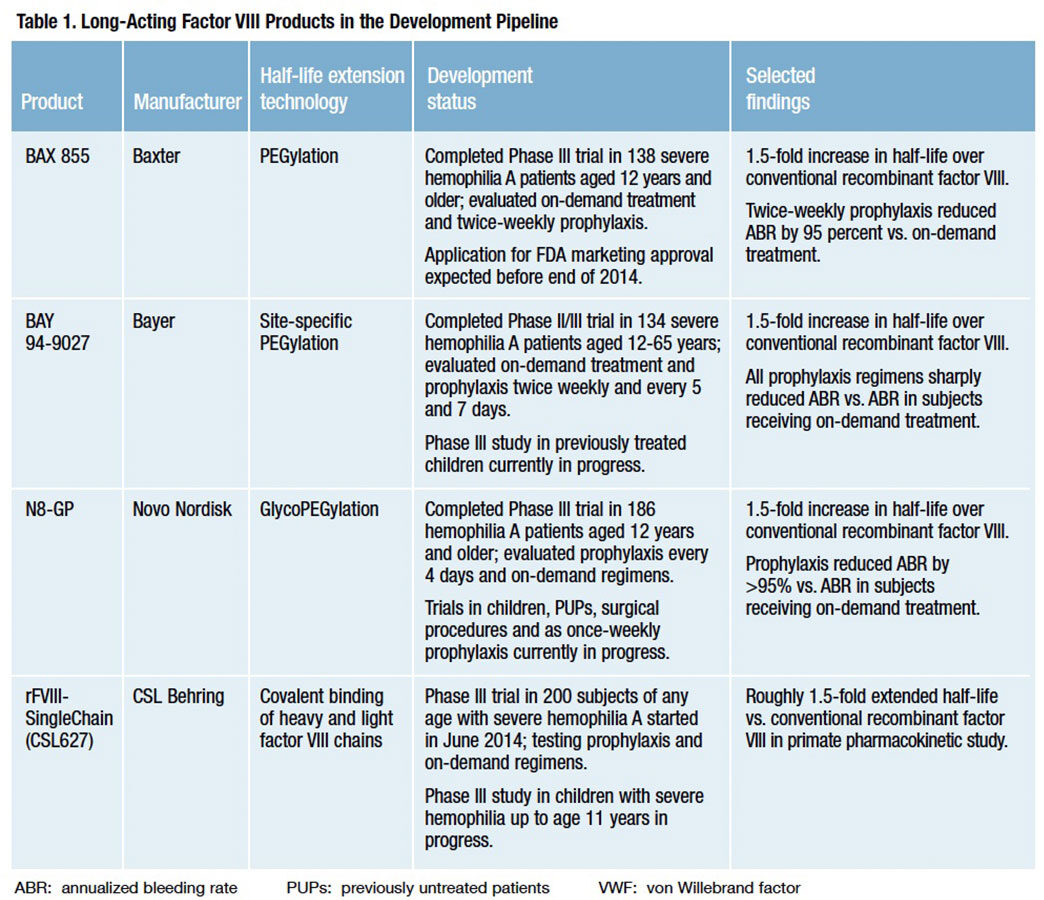
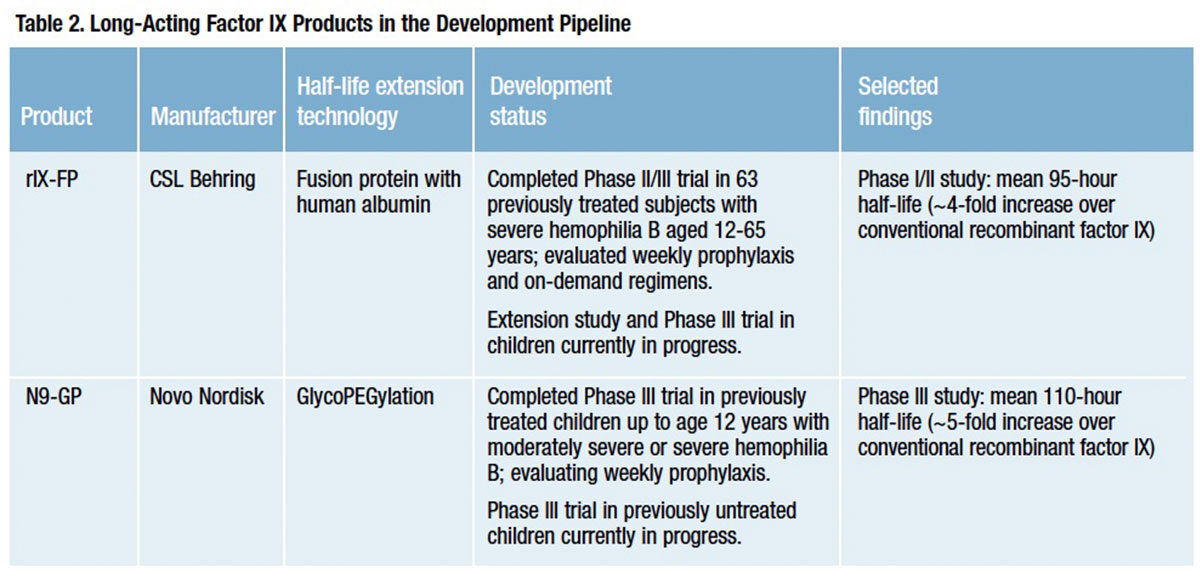
Half-life extension in four of these six products — Novo Nordisk’s “N8-GP,” Baxter’s “BAX 855” and Bayer Pharmaceutical’s “BAY94-9027” factor VIII products and Novo Nordisk’s “N9- GP” factor IX — is achieved by attaching long polymer chains of polyethylene glycol (PEG) to the therapeutic protein via a process of covalent conjugation known as PEGylation, or a variant method called glycoPEGylation. In addition to other benefits, these long PEG polymer strands shield the protein from exposure to proteolytic enzymes and immune clearance mechanisms, thus protecting its functionality and prolonging its intravascular persistence (Figure 2).
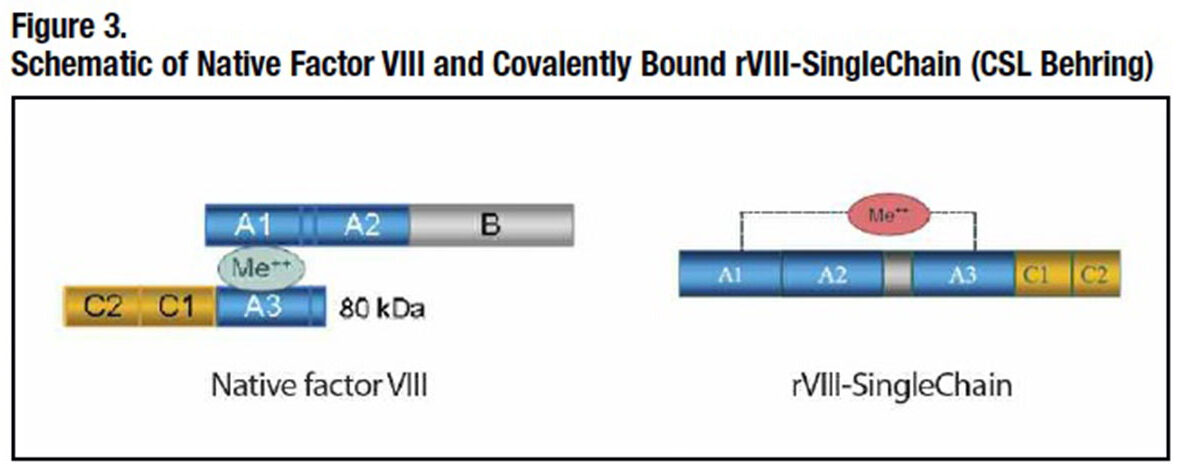
CSL Behring has pursued its own innovative approaches. The company has redesigned recombinant human factor VIII to covalently bond its heavy and light chains together (Figure 3). Its resulting “rVIII-SingleChain” molecule is both more intrinsically stable and has a much higher affinity for von Willebrand factor (VWF), further enhancing its stability in the circulation. CSL Behring’s long-acting recombinant factor IX exploits recombinant albumin fusion technology. Fusing albumin to factor IX creates at least two potential advantages: Albumin has a remarkably long intravascular half-life of about 20 days and, like the Fc portion of IgG1, it is unlikely to elicit unwanted immunogenicity. Albumin fusion technology has appeal as well from the production standpoint: A high-quality product can be manufactured with fewer post-expression modifications and purification steps than PEGylation, and it can be produced more efficiently than other fusion protein approaches, including use of the IgG1 Fc fragment.4 However, while numerous albumin fusion proteins are currently in clinical and preclinical development, to date just one therapeutic applying this technology has been approved for human use.**
Assuming most or all of these investigational products receive FDA marketing approval, we can expect an unusually spirited competition as new entrants strive to win over physicians and their patients. Most safety, pharmacokinetic and efficacy findings reported to date from the four investigational long-acting factor VIII agents are remarkably similar to those for Biogen Idec’s Fc fusion-based ELOCTATE. All appear to have about a 1.5-fold extended circulating half-life compared with conventional recombinant factor VIII. Phase III clinical trial data suggest that optimized prophylaxis with several of these could reduce the risk of breakthrough bleeds by around 95 percent in relation to episodic therapy — again quite similar to ELOCTATE.
No safety-related concerns have been described for any of these six investigational long-acting coagulation factor therapies. With the exception of a single isolated instance, inhibitor antibodies in pivotal clinical trials of these investigational agents have not been reported. It is not unreasonable to expect that physicians and patients could soon be in a position to choose from among four or five long-acting factor VIII products and as many as three long-acting factor IX products.
What Comes Next?
For persons with hemophilia either on or contemplating a prophylaxis regimen, the arrival of this new generation of long-acting clotting factors will translate into a reduced self-infusion burden, much-improved likelihood of treatment compliance, fewer bleeding events, and reduced joint disease and related disability. Fewer units of factor will be infused, but offsetting this will be higher cost per unit.
Will extended half-life factor VIII and IX therapies turn out to be the last important wave of innovation we see from industry until gene therapy someday reduces or eliminates the need for replacement therapy altogether? Don’t count on it. Researchers at Chugai Pharmaceuticals have developed a humanized bispecific monoclonal antibody, named ACE910, designed to mimic the physiologic function of factor VIII by acting as a cofactor that promotes the activation of factor X by factor IXa. Importantly, it has been shown to be phospholipid-dependent, implying that its activity will be limited to the hemostatic site when introduced into the circulation. In a primate model, a single bolus was shown to have potent hemostatic activity equivalent to a therapeutic dose of human factor VIII.5
ACE910 has been in-licensed by Roche, and renamed RG6013. An 82- subject Phase I clinical trial of RG6013 is now in progress in Japan. Preliminary pharmacokinetic, safety and tolerability findings will be presented this December at the annual meeting of the American Society of Hematology in San Francisco. Should this Chugai/Roche factor VIII mimetic prove to be safe and effective, consider these two features: It is dosed subcutaneously and appears to have a half-life of about three weeks.
As much as the extraordinary collaboration of clinical scientists and industry has done to improve health and the quality of life for persons with hemophilia, all indications suggest that more innovation yet lies ahead.
* Factor IX is generally dosed prophylactically two to three times weekly. The sole conventional recombinant factor IX approved for routine prophylaxis (RIXUBIS; Baxter Healthcare) is dosed twice weekly.
** TANZEUM (albiglutide) (GlaxoSmithKline). TANZEUM is a GLP-1 receptor agonist indicated for use in adults with type 2 diabetes. Approved April 2014.
References
- AFREZZA (MannKind Corporation). Full prescribing information. Accessed 8/28/2014 at www.accessdata.fda.gov/drugsatfda_docs/label/2014/022472lbl.pdf.
- The Marketing Research Bureau, Inc. Survey on Hemophilia Care and Price Monitoring – United States (Wave #21). April 2013.
- Czajkowsky DM, Hu J, Shao Z, et al. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 2012;4:2015-28.
- Schmidt SR. Fusion Protein Technologies for Biopharmaceuticals: Applications and Challenges (March 2013). John Wiley & Sons.
- Muto A, Yoshihashi K, Takeda M, et al. Anti-factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost 2014;12:206-13.