New Part B Drug Payment Models
- By Bonnie Kirschenbaum, MS, FASHP, FCSHP
THE PRICE OF drugs, immunologics and biologics and their complicated reimbursement structure have taken a toll on both patients and the healthcare environment. And now, new proposals, models and rules from the Centers for Medicare and Medicaid Services (CMS), the U.S. Food and Drug Administration and the Department of Health and Human Services (HHS) that will affect payments have emerged this spring, necessitating changes for healthcare practices. This column will concentrate on the proposed rule announced by CMS on March 8 that will test six different options for Medicare Part B reimbursement under the outpatient prospective payment system (OPPS).
Under the auspices of the Center for Medicare and Medicaid Innovation (CMMI) created by the Affordable Care Act, CMS has the authority to test innovative payment and service delivery models with the goal of reducing program expenditures under Medicare and Medicaid and at the same time preserving or enhancing the quality of care furnished to individuals under these programs. With this authorization, CMMI will test innovative payment and service delivery models that address a defined population for which there are deficits in care. Successful models will then be expanded to cover more geographic areas over a longer period of time.
Current Medicare Part B Drug Payments under OPPS
Drugs, biologics and radiopharmaceuticals currently are reimbursed by Medicare in one of several ways: as pass-through drugs, as separately payable drugs and as nonseparately payable products that are bundled or packaged into the reimbursement for the service or procedure. Bundling or packaging means there is no separate identified payment for the product, and disbursing the bundled payment is left to the discretion of the facility. Pass-through drugs and separately payable drugs currently are paid at a rate of average sales price (ASP) plus 6 percent (minus approximately 2 percent for as long as sequestration remains in place).
Medicare Part B Drug Payment Model Rule Proposed by HHS
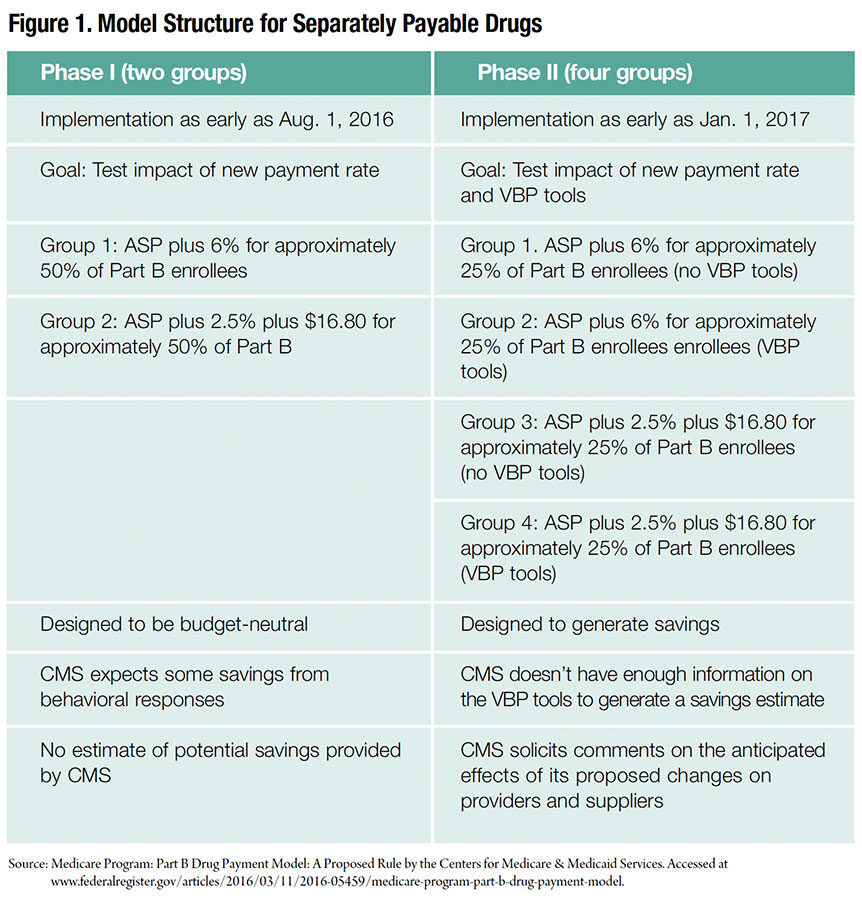
If implemented, the new two-phase proposed rule by HHS would significantly impact how Medicare pays for separately payable Part B program drugs in about half of the states as early as August 1. In phase I, the new formula would change the incentives for prescribing certain drugs, strongly affecting reimbursement and revenues for a wide range of providers. Phase II would introduce “value-based drug pricing” that could further impact prescribing incentives, as well as hospital and physician revenues. (See Figure 1.)
In selecting participants, CMS considered five options for geographic areas in which clusters of providers would be assigned to one of four arms of the model. All providers and suppliers furnishing covered and separately paid Part B drugs would be required to participate if chosen. The proposal uses primary care service areas (PCSAs), which are clusters of ZIP codes that reflect primary care service delivery and are defined and updated under contract to the Health Resources and Services Administration by The Dartmouth Institute. Of the 7,144 PCSAs, 96 in Maryland where hospital outpatient departments operate under an all-payer model would be excluded. The remaining 7,048 PCSAs would be assigned to one arm of the model (the control and three test arms) using a stratified random approach.
The proposed rule would create a demonstration program to test ways to reimburse for separately payable prescription drugs that would largely impact Part B drug reimbursement in physician offices, hospital outpatient clinics and stand-alone clinics that specialize in areas such as oncology or immunology. It would include all Part B drugs and biologics (including biosimilars) with Healthcare Common Procedure Coding System codes.
The following products would be excluded from the model and would continue to be paid for in the current manner:
- Blood and blood products
- Contractor-priced drugs (unless the contractor opted to include them)
- Drugs infused with covered durable medical equipment (DME) (excluded from phase I)
- Drugs in short supply
- Drugs that fall under bundled or packaged payment
- End-stage renal disease drugs
- Influenza, pneumococcal, pneumonia and hepatitis B vaccines
CMS intends to test this program for five years, during which time the agency would monitor the progress and impact of the new payment scheme.
Phase I would recalculate the outpatient Part B payment formula from ASP plus 6 percent to ASP plus 2.5 percent plus a flat fee of $16.80 per drug per day. The start date for phase I would be 60 days following the final rule publication. The flat fee would be updated annually and would be based on the consumer price index for medical care. Sequestration reductions of approximately 2 percent would apply to all payments, including the flat fee.
Phase II, implemented as early as January 2017, would use value-based tools for medication purchases. These tools would include concepts such as reference pricing (an average payment rate based on therapeutically equivalent drugs), indications-based pricing (based on comparative studies), discounting or eliminating patient cost sharing, and clinical decision support (evidence-based).
The rationale behind the design of the first phase of the model is that current payment methodology based on a percentage of cost markup may incentivize physicians to use more expensive drugs when equally effective but less costly ones are available. Whether or not this is the motivation, sites using expensive medications would be most impacted by the proposed model. The second phase would test a variety of value-based purchasing (VBP) strategies that could be beneficial to all sites regardless of specialty.
Using a range of analytical methods, researchers would separately evaluate the impact of each intervention assigned to each model test arm compared with those in areas assigned to the control arm. These key evaluation metrics would include, among others, whether the model resulted in:
- Reduction in Part B drug spending, as well as total Part B and total Medicare program expenditures
- Changes in overall utilization and prescribing patterns and for specific types of providers and suppliers
- Changes in the prices at which providers and suppliers are able to obtain Part B drugs
- Changes in the quality of care, access to care, timeliness of care and the patient care experience
- Observable, unintended consequences
Impact to Practice Sites
The proposed rule would have significant impact upon healthcare sites. Key points are the details of the proposed rule itself and its financial impact. It is likely that OPPS drug reimbursement will be less than the current model for drugs with ASP over $480, and more for drugs with ASP under $480. When performing an analysis for a healthcare site, the finance department must remember to exclude all specifically excluded products and all bundled payment products. This proposal would cover only separately payable Part B products.
Editor’s Note: The content of this column is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.