Reimbursement FAQs: Coding Systems and Other Updates
- By Bonnie Kirschenbaum, MS, FASHP, FCSHP
There’s no doubt that complexity is the operational word for reimbursement, and this includes the rules and nuances that govern drugs and biologicals. This column summarizes some of the additional important issues that deserve attention in 2014. Even though some healthcare practitioners’ sites may contract with an outside provider of billing services, it’s incumbent on the providers and their office staffs to know the background information on the requirements of what’s reimbursable. One of the best sources of information remains MLN Matters publications, a free service provided by the Centers for Medicare & Medicaid Services. See the details for accessing MLN Matters newsletters at the end of the column.
A New Set of Healthcare Common Procedure Coding System (HCPCS) Codes for Drugs, Biologicals and Immunologics
Although for several years HCPCS codes assigned to drugs and biologicals trended with the concept of using a generic description, the advent of newer biologicals and biosimilars has brought the assignment of brand-specific HCPCS codes into play. Not recognizing this and not incorporating this into physicians’ billing for drugs and biologicals will drive a fatal blow to the reimbursement picture! These examples provide some background for practice sites to get started with. A new HCPCS code for Neupogen (filgrastim) was released on Nov. 29, 2013, by the Centers for Medicare & Medicaid Services (CMS) as part of the HCPCS code set updates that became effective Jan. 1, 2014. The new HCPCS code for Neupogen is J1442 injection, filgrastim 1 mcg, and it replaces the old Neupogen HCPCS codes of J1440 for 300 mcgs and J1441 for 480 mcgs. The new code has a billing unit designation of 1 mcg. It’s critical for healthcare providers to make sure billing unit conversion is working in their systems. The dose administered must be converted into billing units to be billed. For example:
Neupogen 300 mcg = 300/1 = 300 billing units of 1 mcg (the single use vial)
Neupogen 480 mcg = 480/1 = 480 billing units of 1 mcg (the prefilled syringe)
Granix (tbo-filgrastim) was approved as a new biologic product with its own labeled indications and not as a biosimilar. Effective Jan. 1, 2014, it has its own HCPCS code (J1446) and its own billing unit designation (5 mcg), as well as its own reimbursement rate and labeled indications. Using the HCPCS code, billing unit designation and applying the reimbursement rate for filgrastim is not appropriate for Granix. Continuing to use a miscellaneous code is not appropriate either and will result in zero reimbursement.
Key points to remember: Neupogen and Granix have different labelled indications, different HCPCS codes and different billing units assigned to them. Healthcare professionals should check their systems carefully to ensure they’ve captured these changes that were effective Jan. 1, 2014.1
ICD-10 Coding System Transition
Several years ago, work began on changing the coding system used to describe diagnoses and procedures. The goal was to build increased complexity and specificity into the code sets. The mandatory launch of the new International Classification of Diseases, Tenth Revision (ICD-10), diagnosis code set, which will replace the current ICD-9 code set published in 1977, was scheduled for Oct. 1, 2014. However, on March 31, the Senate voted to pass a House-approved measure (HR 4302) that would delay the ICD-10 compliance deadline until 2015. As of this writing, it is expected that President Obama will sign the measure.
ICD-10 increases the number of diagnosis codes from 17,000 to 140,000. Although many sites perceive this as a huge and almost insurmountable burden, others are using it as an opportunity and strategic initiative to improve their practice site’s performance under the new system. This clinically driven revenue cycle process will require training on and testing new systems that need to be ramped up in the next few months.Planning for the possible contingencies related to denied or delayed claims and productivity drops need to be factored in as well.
The Centers for Medicare & Medicaid Services has a website dedicated to providing agency-wide information and education on the ICD-10 implementation that includes a video providing a basic introduction to coding for ICD-10 at www.cms.gov/Medicare/Coding/ICD10/index.html?redirect=/ICD10/01_Overview.asp# 2014.
New NDC Codes for Flebogamma 10% DIF Intravenous Immune Globulin (Human)
Recently, Grifols changed its NDC codes for Flebogamma 10% DIF intravenous immune globulin (human) to be in compliance with the U.S. Food and Drug Administration. The new NDC codes are as follow:
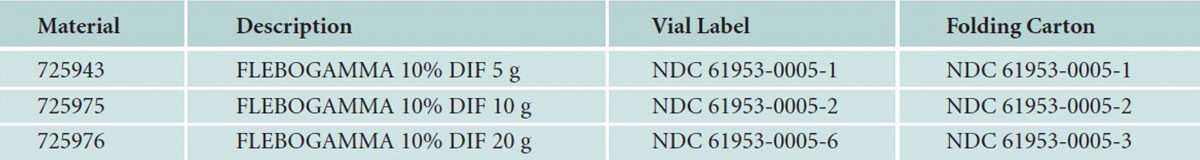
And, as new lots become available, new NDC codes will be assigned.
Mandatory Reporting of 8-Digital Clinical Trial Number
Items or services provided to Medicare patients in clinical trials that qualified for coverage as specified in the Medicare National Coverage Determination Manual (publication 100-03, section 310.1) may be eligible for reimbursement. Although reporting of the clinical trial identifier number has been encouraged on a voluntary basis since 2008, effective Jan. 1, the Centers for Medicare and Medicaid Services’ (CMS) Change Request (CR) 8401 makes it mandatory to report a clinical trial number on these claims. The identifier number is not new; it’s the same one that has been assigned by the National Library of Medicine (NLM) website at clinicaltrials.gov when a new study appears in the NLM Clinical Trials database. However, if providers still don’t have the capacity to locate this number using the Internet, a generic 8-digit number (99999999) may be used only for the balance of 2014 following instructions in CR8401. The fields cannot be left blank; they must be populated for trial-related claims to process appropriately. Specifically, the clinical trial identifier number needs to be included if the beneficiary is enrolled in an approved clinical trial, and the claim is for the investigational item or service, and/or the costs are related to the investigational item or service, and/or the costs are related to routine care for the condition in the clinical trial.2,3,4,5
Annual Clotting Factor Furnishing Fee
Since Jan. 1, 2005, mobile medical application (MMA) rules require that a clotting factor furnishing fee be paid separately if providers furnish clotting factor, unless costs associated with furnishing the clotting factor are paid through another payment system. The Centers for Medicare & Medicaid includes the clotting factor furnishing fee in the published national payment limits for clotting factor billing codes.6 However, when it isn’t included on the ASP Medicare Part B Drug Pricing File or the NOC Pricing File, the providers’ carrier, FI, RHHI or A/B MAC must make payment for the clotting factor, as well as make payment for the furnishing fee. This fee is:
- Calendar year 2013: $0.188 per unit
- Calendar year 2014: $0.192 per unit
Editor’s Note: The content of this column is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
References
- Source: www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/Alpha-Numeric-HCPCS.html
- CMS Manual System Pub 100-04 Medicare Claims Processing: www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R2805CP.pdf.
- Mandatory Reporting of an 8-Digit Clinical Trial Number on Claims: www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM8401.pdf.
- Centers for Medicare & Medicaid Services. Medicare Approved Facilities/Trials/Registries: www.cms.gov/Me d i c a re /Me d i c a re -Ge n e r a l – I n f o rma t i o n /Me d i c a reApprovedFacilitie/index.html.
- MAC toll-free numbers: www.cms.gov/Research-Statistics-Da t a – a n d – S y s t ems /Mo n i t o r i n g – P ro g r ams / p ro v i d e rcompliance-interactive-map/index.html.
- Source: www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/Alpha-Numeric-HCPCS.html