SARS-CoV-2 and COVID-19: Plasma Supply and IG Replacement Therapy to Protect Immunodeficient Patients
Can anti-SARS-CoV-2 antibodies in the plasma supply protect against COVID infection in immunodeficient patients? Research suggests otherwise.
- By Terry O. Harville, MD, PhD
THE GLOBAL PANDEMIC beginning in late 2019 and early 2020 caught nearly everyone off guard and was a wakeup call for developing preparations to protect us all. Since this was the result of a novel zoonotic virus common in bats and now infecting humans, for which humans had not developed immune protection, and since there had not been prior evolutionary exposure to the virus, there was concern about how well the human immune system could respond. Hope was expressed by many that as soon as vaccinations became available and there were survivors of infection, antibodies would become present in donor plasma supply to provide protection for immunodeficient patients. However, I was concerned that this may not be correct.
Anti-ACE2, Anti-Idiotypic Antibodies
SARS-CoV-2 infects cells via binding to ACE2 on the surface of cells via the receptor-binding domain (RBD) region of the spike protein of the virus. Accordingly, we were the first to hypothesize that making antibodies to the RBD region (via vaccination or by infection) would, in turn, result in anti-idiotypic antibodies that would bind to ACE2.1 Further, we were concerned these antibodies could disrupt ACE2 function (regulation of inflammation and blood pressure), which could result in increased inflammation in patients and dysautonomia (dysregulation of blood pressure). We studied this and demonstrated it to be true: People infected with SARS-CoV-2 do make anti-ACE2 antibodies, and these disrupt ACE2 function.2
Subsequently, researchers reported that microvascular clots could be found throughout the tissues in persons infected with SARS-CoV-2. They noted that the anti-ACE2 antibodies could be involved by binding to endothelial cells of the blood vessels, which then would initiate the complement cascade, resulting in damage, which subsequently would initiate the clotting cascade and thereby result in microclotting throughout the body, causing organ dysfunction.3 Other researchers also found anti- ACE2 antibodies, which correlated with neurologic symptoms in post-COVID-19 patients, as well as in some patients after vaccination.4
It is known that the neutralizing antibodies (directed to the RBD region) generated by infection or by vaccination last only a few months, at least in persons with normal functioning immunity. While this may not seem to have a “most direct” effect on the anti-ACE2 antibodies via normal immune system function, the production of anti-ACE2 antibodies could directly affect the presence of the neutralizing anti-RBD antibodies. Figure 1 demonstrates this process: An antibody made to the SARS-CoV-2 spike protein RBD region becomes a new antigen for the immune system to generate an antibody against it. This initial antibody is known as an “idiotype,” and the antibody directed against it is an “anti-idiotype.”
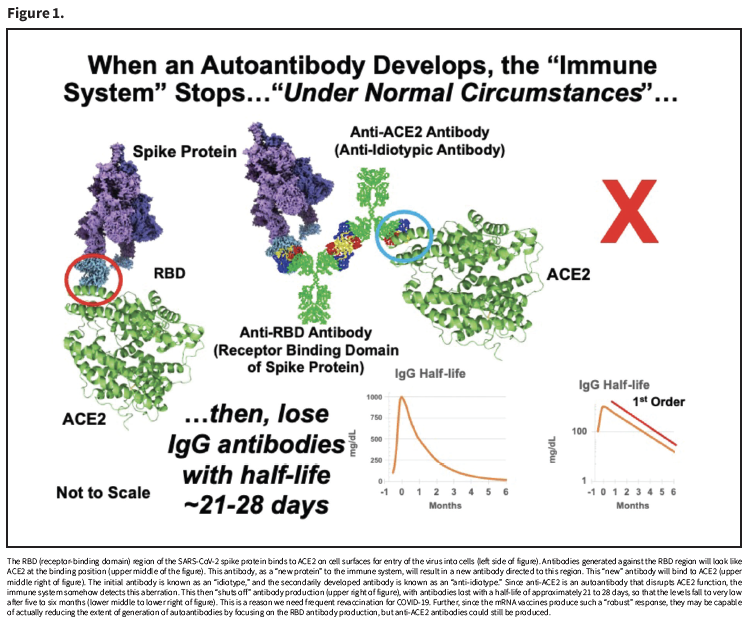
Therefore, an antibody that can bind to the RBD region (idiotype) will “look like” the ACE2 protein (the binding target of the RBD region). So, an antibody to the RBD region-antibody (anti-idiotype) will be capable of binding to ACE2. Under normal immunologic control, this is perceived as “autoantibody production,” which in turn shuts off the antibody production, and antibodies are then lost with a typical half-life of three to four weeks, and expectedly becoming very low after five to six months. Since this is not synchronized across all plasma donors, there is concern that significant amounts of anti-ACE2 antibodies could be in the donor plasma supply at any time.
Autoimmune Antibodies After COVID-19
A group of scientists who were among the first to study and report the presence of autoimmune antibodies in patients after COVID-19 found most people produce more than 1,000 autoantibodies.5 Subsequently, numerous other studies demonstrated a myriad of autoantibodies persisting in people after COVID-19 infection.6-7 Indeed, we have data demonstrating specific autoimmune antibodies directed to central nervous system proteins in patients exhibiting long COVID. Thus, the immune system, not knowing how to correctly respond to this zoonotic virus, ends up generating substantial numbers of autoimmune antibodies. It is of concern that these will become prominent in the donor plasma supply, as more people have survived the infection and donate.
There may be some good news, though. Vaccination has been demonstrated to prevent or reduce the symptoms of long COVID.8 We believe vaccination with an mRNA vaccine guides the immune system in such a strong manner to help redirect it away from autoantibody production, since it is focused on anti-RBD antibody production. As such, this could help reduce the extent of autoantibodies present over time, but we need frequent and ongoing vaccinations to accomplish this! Therefore, if we could prevent natural infections through extensive vaccination, we could effectively reduce the level of autoantibodies in donor plasma. Unfortunately, though, there could still be issues with anti-ACE2 antibodies generated by vaccination.
Post-Infection Convalescent Plasma for Treating COVID-19
Early in the pandemic, there were no vaccines and no antiviral medications for treatment. So, just like the late 1800s concept, “anti-serum” from a prior infected animal or person could be used to treat the current infection in someone. In fact, this has been quite successful, winning the Nobel Prize for Emil von Behring in
1901.9 As early survivors of COVID-19 became available, “convalescent plasma” was obtained for treating patients who were ill with COVID-19. But, early attempts were not very successful.10 It was thought that the anti-viral titer (the amount of anti-SARS-CoV-2 antibodies present) was too low in the convalescent donor plasma, but the reality was that even higher titers of post-COVID-19 convalescent plasma were not that useful. Indeed, there have been meta-analyses demonstrating no overall significant benefit from the use of convalescent plasma to treat COVID-19.11
Further, we know vaccination does not prevent infection from occurring, but reduces the chance of dying.12 This is based on the fact that T lymphocyte immunity from vaccination is quite robust and long-lived, helping to prevent death, whereas antibody production does not seem to provide as much relevant benefit.13 Considering the lack of benefit from convalescent plasma and the fact that vaccine-induced antibodies or prior infection-induced antibodies do not prevent infection, it is not clear whether anti-SARS-CoV-2 antibodies in immune globulin (IG) replacement therapy actually provide the typically expected benefit of antibodies to patients.
Replacement IG Recalls
Anti-SARS-CoV-2 antibodies were reported to be detected in the donor plasma for intravenous IG (IVIG) and subcutaneous IG (SCIG) production late in 2020.14 Since 2022, there have been at least 12 recalls of IVIG and SCIG due to unexpected reactions.15 While these recalls have not directly been reported to be due to antibodies related to COVID- 19, the timing is highly suggestive that plasma donors who had prior COVID-19 infection have become significant plasma donors, with the implication that their autoimmune antibodies are in the donor plasma supply. As such, adverse reactions may be expected to occur.
Discussion
SARS-CoV-2 presented as a novel zoonotic virus for which our immune system had no prior knowledge or recourse. As a consequence of how the virus infects cells, anti-ACE2 antibodies are generated. Additionally, after COVID-19 infection, patients may develop more than 1,000 autoantibodies. Microclotting is a severe consequence of having anti-ACE2 antibodies and autoantibodies, with studies suggesting these can result in multiple tissue and organ dysfunction and, importantly, brain injury.
Anti-SARS-CoV-2 antibodies do not prevent infections from occurring, and data presented have not demonstrated benefit against COVID-19 infection, but vaccination (and natural infection) do result in T lymphocyte anti-SARS-CoV-2 function, which can prevent death. Recurrent vaccination with mRNA vaccines may be helpful for reducing the amount of autoantibodies by focusing the immune system on anti-RBD antibody production and redirecting it from autoantibody production, but at the risk of production of anti-ACE2 antibodies.
Therefore, while many have been hoping for anti-SARS-CoV-2 antibodies to become prominent in the plasma supply for protecting immunodeficient patients, I have remained concerned that the co-occurring autoimmune antibodies may actually be detrimental. And now, the reduction in access to COVID-19 mRNA vaccines may exacerbate the situation.
References
- Harville, TO, and Arthur, JM. Anti-Idiotypic Antibodies in SARS-CoV-2 Infection and Vaccination. New England Journal of Medicine, 386(5):February 2, 2022. Accessed at www.nejm.org/doi/full/10.1056/NEJMc2119443.
- Arthur, JM, Forrest, JC, Boehme, KW, Kennedy, JL, Owens, S, Herzog, C, Liu, J, and Harville, TO. Development of ACE2 Autoantibodies After SARS-CoV-2 Infection. PLoSONE, 16(9):e0257016. Accessed at journals.plos.org/plosone/article?id=10.1371/journal.pone.0257016.
- Lee, MH, Perl, DP, Steiner, J, Pasternack, N, et al. Neurovascular Injury with Complement Activation and Inflammation in COVID-19. Brain, 2022 Jul 29;145(7):2555-2568. Accessed at pubmed.ncbi.nlm.nih.gov/35788639.
- Bellucci, M, Bozzano, FM, Castellano, C, et al. Post-SARS-CoV-2 Infection and Post-Vaccine-Related Neurological Complications Share Clinical Features and the Same Positivity to Anti-ACE2 Antibodies. Frontiers in Immunology, 2024 Aug 1;15:1398028. Accessed at pubmed.ncbi.nlm.nih.gov/39148725.
- Wang, EY, Mao, T, Klein, J, et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature, 2021;595:283–288. Accessed at www.nature.com/articles/s41586-021-03631-y.
- Rojas, M, Rodríguez, Y, Acosta-Ampudia, Y, et al. Autoimmunity Is a Hallmark of Post-COVID Syndrome. Journal of Translational Medicine, 2022;20:129. Accessed at pubmed.ncbi.nlm.nih.gov/35296346.
- Talwar, S, Harker, JA, Openshaw, PJM, and Thwaites, RS. Autoimmunity in Long COVID. Journal of Allergy and Clinical Immunology, 155(4):1082-1094. Accessed at pubmed.ncbi.nlm.nih.gov/39956285.
- Byambasuren, O, Stehlik, P, Clark, J, Alcorn, K, and Glasziou, P. Effect of Covid-19 Vaccination on Long Covid: Systematic Review. BMJ Medicine, 2023 Feb 1;2(1):e000385. Accessed at pubmed.ncbi.nlm.nih.gov/36936268.
- von Behring, E, and Kitasato, S. Ueber das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren Layoutgetreues Digitalisat der Ausg.: 1890 Standort: Fachgebiet für Geschichte der Medizin (192) Signatur: EvB/SD 1 Bemerkungen: Sonderdruck aus Deutsche Medizinische Wochenschrift. 1890, Nr. 49 Provenienz. Accessed at archiv.ub.uni-marburg.de/ubfind/Record/urn:nbn:de:hebis:04-eb2013-0164/Description#tabnav.
- Korley, FK, Durkalski-Mauldin, V, Yeatts, SD, et al. Early Convalescent Plasma for High-Risk Outpatients with Covid-19. New England Journal of Medicine, 2021 Nov 18;385(21):1951-1960. Accessed at www.nejm.org/doi/full/10.1056/NEJMoa2103784.
- Filippatos, C, Ntanasis-Stathopoulos, I, Sekeri, K, et al. Convalescent Plasma Therapy for COVID-19: A Systematic Review and Meta- Analysis on Randomized Controlled Trials. Viruses, 2023 Mar 16;15(3):765. Accessed at pubmed.ncbi.nlm.nih.gov/36992474.
- Ioannidis, JPA, Pezzullo, AM, Cristiano, A, and Boccia, S. Global Estimates of Lives and Life-Years Saved by COVID-19 Vaccination During 2020-2024. JAMA Health Forum, 2025;6(7):e252223. Accessed at jamanetwork.com/journals/jama-health-forum/fullarticle/2836434.
- Wang, L, Nicols, A, Turtle, L, et al. T Cell Immune Memory After Covid-19 and Vaccination. BMJ Medicine, 2023 Nov 22;2(1):e000468. Accessed at pubmed.ncbi.nlm.nih.gov/38027416.
- Romero, C, Diez, JM, and Gajardo, O. Anti-SARS-CoV-2 Antibodies in Healthy Donor Plasma Pools and IVIG Products. The Lancet Infectious Diseases; 21(6):765–766. Accessed at www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00059-1/fulltext