Surviving Sepsis: It’s Time to Put Albumin to the Test
- By Keith Berman, MPH, MBA
“We recommend crystalloids be used as the initial fluid of choice in the resuscitation of severe sepsis and septic shock.”
— 2012 Surviving Sepsis International Guidelines for Management of Severe Sepsis and Septic Shock
“Until additional data are available, clinicians may consider albumin as a first-line resuscitation fluid for patients with sepsis.”
— The SAFE Investigators (2011)
SPREAD OVER THE last 15 years are a pair of landmark trials and a number of smaller studies whose results suggest that use of human albumin as the initial resuscitation fluid in patients with severe sepsis or septic shock can importantly reduce its stubbornly high 30 percent death rate.
In a 1999 randomized trial of 126 patients with cirrhosis and spontaneous bacterial peritonitis — a condition that shares many features with the sepsis syndrome — mortality during hospitalization was dramatically lower in those who received albumin in addition to cefotaxime (10 percent vs. 29 percent, p = 0.01).1 Then, in 2004, the Saline versus Albumin Fluid Evaluation (SAFE) study, organized by publicly financed hospitals in Australia and New Zealand, uncovered something unexpected: A subgroup analysis of more than 1,200 ICU patients with severe sepsis revealed a relative risk of death of 0.89 associated with resuscitation solely with 4% albumin instead of saline.2 As this strong trend did not reach statistical significance, there were numerous calls for further clinical research.
Yet, here we find ourselves today with no new clinical research, no robust data to answer whether and to what extent albumin use may reduce mortality. The current Surviving Sepsis guideline continues to recommend crystalloids as the initial resuscitation fluid of choice. Albumin is suggested only for patients who “require substantial amounts of crystalloid,” citing “the absence of any clear benefit following the administration of colloid solutions compared to crystalloid solutions” in this population.3
But, meanwhile, recent new evidence has added weight to the hypothesis that albumin resuscitation instead of crystalloids can reduce the mortality burden from severe sepsis.
Albumin Does — and Doesn’t — Spare Lives in Severe Sepsis
Meta-analysis of 17 sepsis trials. Seven years after publishing their landmark study, the SAFE investigators conducted a systematic review and meta-analysis to further explore whether albumin used in lieu of saline or other resuscitative fluids might confer a survival advantage in patients with sepsis.4 Seventeen studies met the inclusion criteria. Overall, the use of albumin was associated with a reduction in mortality with a pooled estimate of the odds ratio of 0.82 (95 percent confidence interval [CI] 0.67 to 1.0, p = 0.047). Omitting the SAFE study still yielded a similar odds ratio of 0.84 (CI 0.59 to 1.18, p = 0.31). But further, separating eight small studies (totaling 383 participants) using concentrated albumin solutions from nine other studies (totaling 1,594 participants) that evaluated physiologic 4% to 5% albumin solutions revealed a sharp disparity in the effect of albumin concentration on mortality risk:
- Concentrated (≥20 percent) albumin solutions: A non-significant odds ratio of 1.08 favoring saline and other nonalbumin solutions (95% CI 0.7 to 1.68, p = 0.73). A number of these, as well as other very recent trials,5,6 stipulated very large doses or a rigid dosing regimen (e.g., dosing to a target circulating albumin level), independent of hemodynamic considerations.
- Physiologic (4% to 5%) albumin solutions: An odds ratio of 0.76 favoring iso-oncotic albumin solutions over saline and other non-albumin solutions with borderline statistical significance (95% CI 0.61 to 0.95, p = 0.02). A survival advantage associated specifically with use of low-concentration albumin has also been documented in recent mouse models of severe sepsis, suggesting a dose-dependent effect.7
“The results of this meta-analysis suggest that resuscitation with albumin may result in lower mortality compared with resuscitation with other fluids. Until additional data are available, clinicians may consider albumin as a first line resuscitation fluid for patients with sepsis,” the SAFE investigators concluded.
SAFE findings re-examined: a larger mortality treatment effect favoring albumin. In a post-hoc analysis of the severe sepsis subgroup, the SAFE investigators conducted a multivariate logistic regression analysis in 919 of the 1,218 patients for whom there were complete baseline data.8 While assignment to albumin instead of saline was independently associated with a decreased odds ratio for death of 0.87 (95% CI 0.74 to 1.02, p = 0.09), after adjustment for baseline characteristics, the odds ratio favoring albumin resuscitation further decreased to 0.71 (95% CI 0.52 to 0.97, p = 0.03).
A second sub-analysis revealed that this impressive mortality reduction was very similar in albumin recipients whose pre-treatment baseline serum albumin concentration was ≤25 g/L or >25 g/L. If simple blood volume expansion is presumptively the key therapeutic effect of albumin in severe sepsis, why would more hypoalbuminemic patients not experience a larger mortality reduction benefit than those with relatively high baseline serum albumin levels? This similar mortality reduction trend — independent of baseline serum albumin level — suggests that albumin could be mediating pharmacologic actions entirely apart from its colloid properties.
Albumin Italian Outcome Sepsis (ALBIOS) study. In this newly published open-label study,9 100 Italian ICUs randomly assigned 1,818 patients with severe sepsis or septic shock to receive crystalloids only, or crystalloids plus 20% albumin on a daily basis, with the objective of maintaining serum albumin at 3.0 g/dL for 28 days or until ICU discharge. At 28 days after randomization, overall mortality in the two groups was the same — 32 percent and 31.8 percent. At 90 days, there was a small nonsignificant reduction in mortality (41.1 percent vs. 43.6 percent mortality) in the albumin group. A post hoc subgroup analysis of 1,121 patients in septic shock revealed that the relative risk of mortality was 0.87 favoring the albumin treatment group (95 percent CI, 0.77 to 0.99) at 90 days.
The ALBIOS investigators concluded that “the findings in our trial may appear to contradict those of the predefined subgroup analysis from the SAFE study, which suggested a survival advantage with an albumin-based strategy during severe sepsis.” In reality, ALBIOS tested an entirely different hypothesis and a radically different dosing regimen from that of the SAFE study. ALBIOS trialists were rigidly required to dose 20% albumin to reach an arbitrary 3.0 g/dL serum level to address hypothetical “hypoalbuminemia” — regardless of the patient’s hemodynamic status — for up to 28 days. On each day a patient’s serum albumin level fell below 25 g/L, 300 mL of 20% albumin — the oncotic equivalent of 1,200 mL of 5% albumin — was infused.* Through day six, two-thirds of patients in the albumin group were still receiving large daily infusions of concentrated albumin, together with substantial volumes of crystalloids.
By contrast, blinded SAFE study clinicians decided when and how much fluid (albumin or saline) to administer based on each patient’s clinical status and response to treatment. Following standard goal-directed sepsis management guidelines, SAFE study clinicians rapidly tapered their administration of albumin over the first three ICU days:
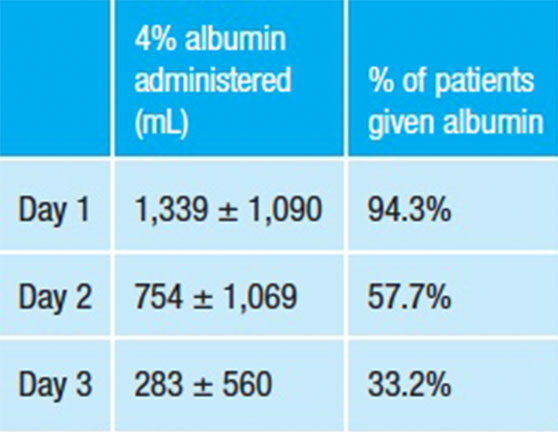
Could repeated infusions of concentrated albumin by ALBIOS trialists to attain an arbitrary target serum level in all patients have adversely affected some patients, potentially countering its hemodynamic or other benefits in others? While this is not an answerable question, what is clear is that the ALBIOS results do not provide much insight into the life-saving potential of 5% albumin used early, appropriately and exclusively for early resuscitation of severe sepsis, in accordance with standard goal-directed principles of resuscitative fluid therapy.
Albumin in Sepsis Resuscitation: Costly or Cost-Effective?
Human albumin comprises more than one-half of plasma protein content in the circulation and performs an array of important physiologic functions. But confined to a bottle or flexible container, it is widely regarded as just another “volume expander.” Even today, most specialists relegate albumin to the catch-all “resuscitative fluids” category occupied by balanced electrolyte solutions, hydroxyethyl starch products and lowly dollar-a-bag saline.
As long it is generally thought of and categorized as a simple resuscitative fluid, 5% albumin will continue to be perceived as an “expensive” alternative to crystalloids for resuscitation of severe sepsis and septic shock. Unsurprisingly, it is hard to find a published review paper, commentary, research article discussion that does not reference the “high cost” of albumin in considering resuscitative fluid options.
But suppose a larger trial were to actually confirm that resuscitation of severe sepsis patients with 5% albumin reduces mortality to a similar extent as the severe sepsis subgroup in the SAFE study? Would albumin resuscitation therapy still be “expensive” in relation to initial resuscitation with lower-cost saline? The answer can be found by simply modeling cost-effectiveness. In this scenario, the cost per life saved by using albumin in lieu of saline as the initial resuscitative fluid is around $7,000 (Table 1). The cost per quality-adjusted life year (QALY) would, of course, come in substantially lower. If one were to apply the adjusted odds ratio after considering differences in baseline factors (0.71),8 which further favors albumin, the cost per life saved and cost per QALY go lower yet.
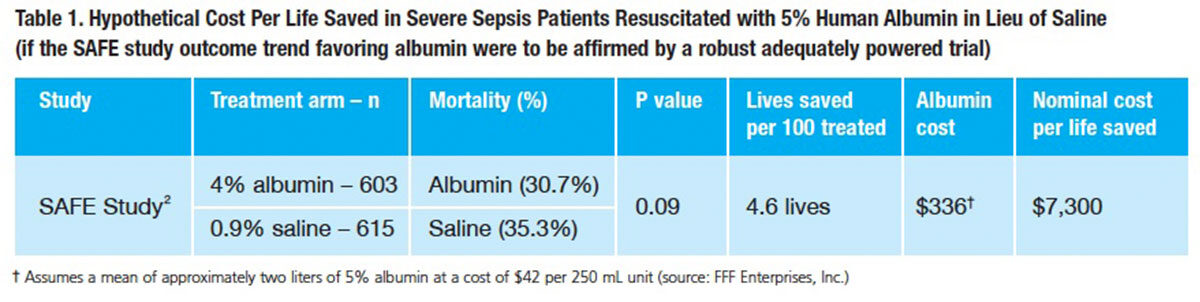
When albumin is examined as a potentially life-sparing therapeutic modality in this treatment setting, cost concerns based on comparing its per-liter cost to the cost of saline are obviously misplaced. The estimated cost per life saved, both in absolute terms and relative to virtually any known life-saving treatment, is so low that it needs no further comment. Assuming a well-designed, adequately powered trial ultimately corroborates the SAFE study findings, albumin resuscitation as a means to reduce the death toll from severe sepsis would be remarkably cost-effective by any measure.
Albumin: From Fluid to Multifunctional Protein Therapeutic
Accumulating evidence now suggests that albumin is a human biologic with a spectrum of physiologic functions that may help protect and restore organ function and improve survival through mechanisms entirely unrelated to its role in regulating fluid compartmentalization:
- As the most abundant extracellular antioxidant in the human body, albumin functions as a potent antioxidant and free radical scavenger.
- Albumin binds and transports numerous endogenous and exogenous substances (e.g., bilirubin, hormones, metal ions, free fatty acids and enzymes), variously facilitating their physiologic function, detoxification and antioxidant protection.
- Albumin regulates microvascular permeability and supports endothelial stabilization.
- Albumin mediates anti-inflammatory activity.
Sepsis induces a complex inflammatory response, where severe oxidative stress, free radicals, endothelial dysfunction and other factors collectively can lead to multiple organ failure and death. Albumin is a potent multifunctional biologic that happens also to be the most abundant plasma protein. The prospect that there may be important survival benefit from administering iso-oncotic albumin to a severe sepsis patient whose circulating albumin level is low or whose functionality is overwhelmed by the disease process is certainly not far-fetched.
A Golden Research Opportunity Awaits
Albumin is supplied by five manufacturers in the United States. It is a generic, low-priced biologic that is costly to produce. Either individually or acting collectively, these manufacturers simply cannot justify investing millions of dollars in a very large multicenter sepsis trial to try to confirm the strongly suggestive SAFE findings.
A decade ago, Australian and New Zealand government health authorities stepped up to conduct the 7,000-subject SAFE study. The SAFE investigators and numerous commenters have subsequently called for “further study” of the role of albumin resuscitation specifically in severe sepsis.
The U.S National Institutes of Health (NIH) annually disseminates $30 billion to support medical research. With the prospect to finally answer whether initial resuscitation with 5% albumin can meaningfully reduce the one-in-three death toll still exacted by severe sepsis and septic shock, is this not a golden opportunity for a team of investigators to design and seek NIH support for a U.S. prospective multicenter study? Inarguably, those would be research dollars well spent.
References
- 1. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. New Engl J Med 1999;341:403-9.
- The SAFE study investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. New Engl J Med 2004;350:2247-56.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013 Feb;41(2):580-637.
- Delaney AP, Dan A, McCaffrey J, et al. The role of albumin as a resuscitation fluid for patients with sepsis: A systematic review and meta-analysis. Crit Care Med 2011 Feb;39(2):386-91.
- Volume Replacement with Albumin in Severe Sepsis (ALBIOS). Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico. Accessed at: www.clinicaltrials.gov/ct2/show/NCT00707122?term=ALBIOS&rank=1.
- Early Albumin Resuscitation During Septic Shock (EARRS). Laboratoire Français de Fractionnement e de Biotechnologies. Accessed at: www.clinicaltrials.gov/ct2/show/NCT00327704?term=albumin+AND+sepsis&rank=24.
- Kremer H, Baron-Menguy C, Tesse C, et al. Human serum albumin improves endothelial dysfunction and survival during experimental endotoxemia: concentration-dependent properties. Crit Care Med 2011 Jun;39(6):1414-22.
- The SAFE Study Investigators. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med 2011;37:86-96.
- Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. New Engl J Med 2014 Mar 18; published online ahead of print. Accessed at www.nejm.org/doi/full/10.1056/NEJMoa1305727.