Thirty Years On, IVIG Clinical Research Keeps Rolling
- By Keith Berman, MPH, MBA
JUST MONTHS AFTER the first commercial intravenous immune globulin (IVIG) product was introduced in 1981, an expert workshop was convened to review its “use and potential.” In addition to reports on the use of IVIG in heart and bone marrow transplant patients, presenters addressed its protective benefits and dosing issues for patients with primary immunodeficiency disorders.1 And that was it.
So it is difficult to imagine that, more than 30 years later, those workshop participants could have foreseen that IVIG remains the subject of intensive clinical research interest, with published case reports, case series and controlled clinical trials now numbering in the thousands. Or that in 2014, IVIG products are being evaluated in dozens of U.S. and international clinical trials for hard-to-treat disorders as diverse as scleroderma and sickle cell crisis.
It has long been appreciated that IVIG preparations, purified from large pools of donor human plasma, contain a broad range of “immune” IgG antibodies directed against bacteria and other pathogens. IVIG, as well as subcutaneously administered immune globulin (SCIG), continues to provide effective long-term passive immunity to protect children and adults with primary and selected secondary humoral immunodeficiency disorders.
But the secret to the breadth and longevity of research interest in IVIG largely lies elsewhere: the product’s vast repertoire of “natural” IgG antibodies that collectively comprise as much as 95 percent of total IgG in our circulation and extracellular compartment. These natural antibodies have a multiplicity of key immunoregulatory functions, including but not limited to:
• Interference with autoantibody production
• Neutralization of self-directed autoantibodies
• Downregulation of macrophage and other phagocyte activity
• Attenuation of inflammation through known mechanisms that include modulation of cytokine production and binding to activated complement components
• Interference of the interaction between T cells and antigen-presenting cells
Added to this are IgG antibodies that appear to be ubiquitous in human blood serum, but whose functions are not well understood. As with innately produced “natural” IgM autoantibodies whose function appears to involve clearing tissues of post-apoptotic cellular debris, we are born with many thousands of natural IgG autoantibodies that are multiply reactive with self-antigens on cells that mediate immunity, as well as other antibodies. IgG autoantibodies were thought to be solely involved with the etiology of autoimmune diseases. But it is now evident that IgG, as well as IgM, autoantibodies are purposefully produced by the healthy immune system to help regulate itself.
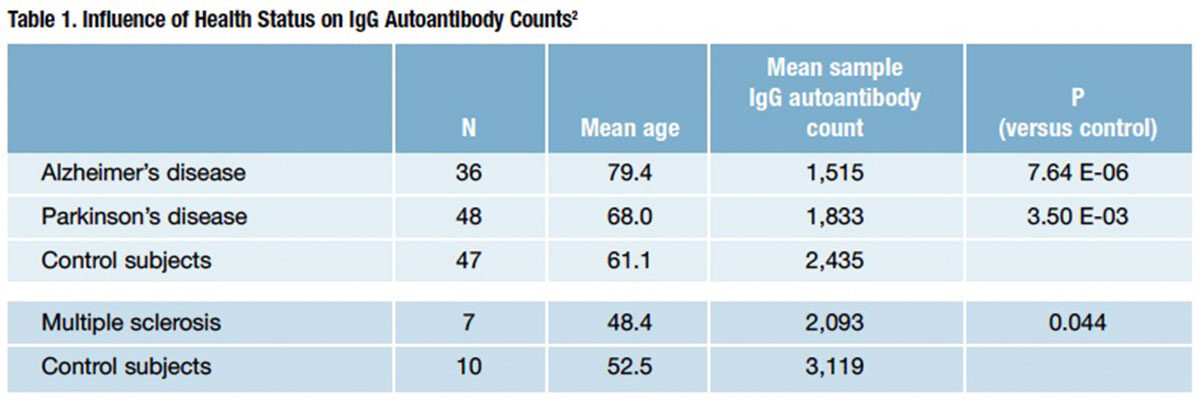
IgG autoantibody counts have been shown to rise with advancing age. However, in a recent study examining persons with three major autoimmune neurological disorders — Alzheimer’s disease (AD), multiple sclerosis (MS) and Parkinson’s disease (PD) — a U.S. investigative team found that IgG autoantibody diversity was significantly diminished — not increased — in each of those populations as compared with healthy control subjects (Table 1).2 Adding to the puzzlement is the fact that these observed drops in IgG autoantibody count in AD, MS and PD directly conflict with a pattern of increasing IgG autoantibody count with age in the general population.
These and innumerable other unanswered questions underscore how little we know about the role of natural human IgG antibodies both in the regulation of normal immunity and in the suppression of autoimmunity.
What’s Hot in IVIG Clinical Research
The decades-long interest in the clinical applicability of IVIG has undoubtedly been fueled by growing awareness of the diverse roles of IgG in immunoregulation, and further abetted by reliance on use of toxic immunosuppressive drugs that reflects our limited understanding of the underlying pathophysiology of most human autoimmune disorders. But perhaps the biggest reason behind the excitement over IVIG is the results themselves: an ever-expanding body of evidence that already has affirmed or strongly suggested health outcome benefits of IVIG administration in a remarkably diverse range of difficult-to-treat autoimmune and inflammatory disorders.
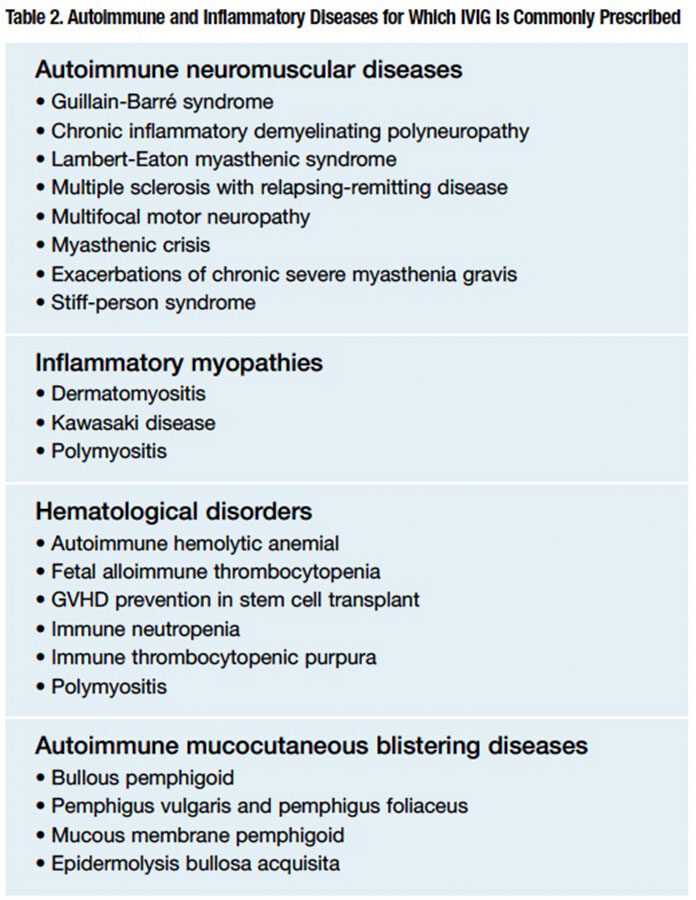
To date, controlled trials have established beneficial health outcomes of IVIG for at least a dozen autoimmune and inflammatory disorders. Based on extensive reported case experience, IVIG is also widely prescribed in numerous other autoimmune neuropathies, neuro-muscular junction disorders, hematological disorders, inflammatory myopathies, mucocutaneous blistering diseases and solid-organ transplant-related disorders (Table 2).
Among many clinical trials currently in progress are several that stand out for their potential to expand the therapeutic utility of IVIG:
Vaso-occlusive crisis in sickle cell disease (SCD). Vaso-occlusive crisis is the most common complication of SCD, and can progress to life-threatening acute chest syndrome. The most affected 5 percent of SCD patients experience from three to 10 crisis episodes per year.3 Other than opioid analgesics to blunt pain resulting from microvascular occlusion, there is currently no specific treatment for this condition.
In a mouse model of SCD, commercial IVIG preparations have been shown to rapidly reduce adherent leukocyte numbers and dramatically inhibit interactions between red blood cells and white blood cells, resulting in improved microcirculatory blood flow and survival rates.4
Encouraged by these animal study findings, researchers at Montefiore Medical Center in New York City initiated a Phase I/II randomized, masked, double-blind clinical trial several years ago, with an enrollment goal of 60 patients between ages 12 years and 65 years who require hospital admission for a pain episode. Patients will receive either a single dose of up to 800 mg/kg of IVIG or normal saline. Primary and secondary endpoints include duration of the pain crisis and total opioid use, respectively. The study is on schedule to complete data collection for the primary outcome assessment in July 2014.
Idiopathic cardiomyopathy. Persistence of parvovirus B19 has been associated with progressive cardiac dysfunction leading to idiopathic cardiomyopathy (ICM). A 2010 pilot study evaluating high-dose IVIG (2 g/kg) therapy in 17 consecutive patients with ICM, symptomatic heart failure and biopsyproven endocardial parvovirus B19 (PVB19) documented a significant improvement in ejection fraction from a pretreatment baseline of 34 ± 3 percent to 41 ± 3 percent six months after IVIG administration.5
This same team of Dutch investigators is currently about midway through a randomized, double-blind placebocontrolled trial designed to evaluate the same dose of IVIG (divided over four days) in 50 patients with chronic symptomatic ICM and a high biopsy-confirmed PVB19 load. The estimated primary completion date is November 2015.
Systemic sclerosis (systemic scleroderma). In addition to skin thickening resulting from vascular injury and subsequent fibrosis, patients with the diffuse form of systemic scleroderma are at risk of developing widespread, severe internal organ involvement. Spontaneous improvement is very uncommon, and current immunosuppressive drug options targeting kidney, lung, skin and other organ diseases are of very limited value.
Encouraged in part by a 100 percent complete or partial response rate to high-dose IVIG in patients with scleromyxedema, a very rare scleroderma-like fibrosing disorder,6 investigators at Johns Hopkins and Georgetown University hospitals initiated a three-to-one randomized, albumin placebo-controlled, double-blind trial in April 2013 to evaluate a six-month course of monthly high-dose (2 g/kg) IVIG in 24 patients with diffuse systemic sclerosis. Effects on skin will be the primary outcome measure; other measured outcomes will include pulmonary function, as well as muscle, joint, inflammatory and various laboratory testing parameters. Findings from a very recent study by Japanese researchers offer evidence that multiple courses of IVIG may be needed to achieve efficacy in many patients with the diffuse form of the disease.7
Bullous pemphigoid (BP). A large majority of adult BP patients (33 of 41) in a recent literature review experienced rapid partial or complete response to IVIG therapy after failure or intolerance to standard immunosuppressive drug therapy. It also appears that a shorter disease duration prior to IVIG therapy strongly correlated with a shorter time to complete response.8
A Japanese Phase III study completed in September 2013 took the next logical step by randomizing 56 patients with BP unresponsive to corticosteroids to receive high-dose IVIG (400 mg/kg/day for five days) or placebo. Potential benefits could include more rapid induction of remission, more durable remission and reduction in corticosteroid- related adverse events. Available evidence together with findings from this first randomized controlled trial of IVIG in BP may prompt a re-evaluation of whether, as has recently been proposed for pemphigus,9 addition of IVIG should be considered earlier in the management of BP and other mucocutaneous blistering diseases.
Yesterday, Today and Tomorrow: A Cornucopia of IVIG Research
The functionality and excellent safety and tolerability of IVIG will continue to attract interest among clinicians concerned with poor efficacy, relapse and adverse events associated with corticosteroids and cytotoxic drugs still relied on as first-line therapy against most autoimmune disorders. Where past studies have failed to confirm case reports of significant benefit for some conditions, could there be overlooked disease subcategories that respond well to IVIG, warranting a new trial or an evaluative course of therapy strategy? Might more aggressive or extended dosing in certain conditions potentially deliver the quantity of deficient immunomodulatory IgG antibodies needed to overcome autoimmune and inflammatory dysregulation?
Thirty years after its quiet introduction, unabated global research interest in IVIG reflects just how much remains unknown about its therapeutic potential. But that should be little wonder when one stops to consider that IVIG — quite literally three-quarters of our humoral immune system supplied in small glass vials —is like no other therapeutic ever introduced into the medical armamentarium.
References
- Intravenous immune globulin: its use and potential. Proceedings of a workshop. San Francisco, Calif., Nov. 23, 1981. J Clin Immunol 1982 Apr;2(2 Suppl):1S-48S.
- Nagele EP, Han M, Acharya NK, et al. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS ONE 2013 Apr 2;8(4):e60726.
- Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. New Engl J Med 1991;325:11-6.
- Chang J, Shi PA, Chiang EY, et al. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood 2008 Jan 15;111(2):915-23.
- Dennert R, Venthius S, Schalla S, et al. Intravenous immunoglobulin therapy for patients with idiopathic cardiomyopathy and endomyocardial biopsy-proven high PVB19 viral load. Antivir Ther 2010;15(2):193-201.
- Blum M, Wigley FM and Hummers LK. Scleromyxedema: a case series highlighting long-term outcomes of treatment with intravenous immunoglobulin (IVIG). Medicine (Baltimore) 2008 Jan;87(1):10-20.
- Takehara K, Ihn H and Sato S. A randomized, double-blind, placebo-controlled trial: intravenous immunoglobulin treatment in patients with diffuse cutaneous systemic sclerosis. Clin Exp Rheumatol 2013 Mar-Apr;31(2 Suppl 76):151-6.
- Gaitanis G, Alexis I, Pelidou S-H, et al. High-dose intravenous immunoglobulin in the treatment of adult patients with bullous pemphigoid. Eur J Dermatol 2012;22(3):363-9.
- Leventhal JS and Sanchez MR. Is it time to re-evaluate the treatment of pemphigus? J Drugs Dermatol 2012 Oct;11(10):1200-6.