Measles: A Patient’s Perspective
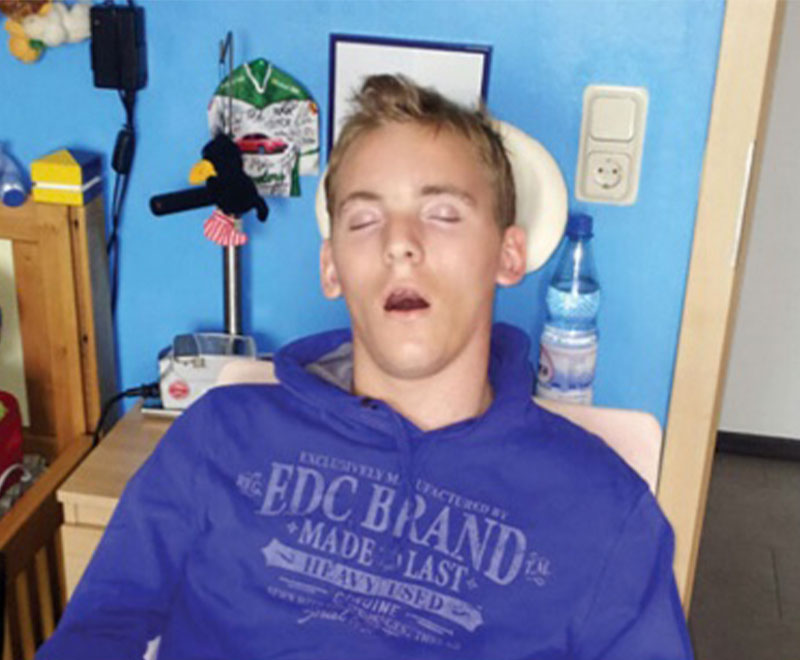
When their youngest son Max became ill with measles in 1995, Rüdiger Schoenbohm and his wife, Anke, were naturally concerned. Max was only 6 months old and still too young to have been vaccinated.
New Data Show One in 31 Kids Has Autism

Autism prevalence in the U.S. has increased from one in 36 children to one in 31, according to the Centers for Disease Control and Prevention’s (CDC) latest Autism and Developmental Disabilities Monitoring (ADDM) Network survey.
COVID-19 May Increase IVIG-Resistance in Kids Prior to Developing Kawasaki Disease

A recent study found there is significantly higher intravenous immune globulin (IVIG) resistance among children who contracted COVID-19 before developing Kawasaki disease
Measles: A Physician’s Perspective

George Rust, MD, MPH, joined the faculty of the Florida State University (FSU) College of Medicine in 2016 and serves as medical director for six local county public health department. He is the author of a 2025 measles fact sheet to help address this important public health concern.
Activating B Cells Key to TIL Cancer Therapy Effectiveness
Researchers at Moffitt Cancer Center have found that tapping into the body’s own immune system and activating a type of immune cell known as B cells could be the key to boosting the effectiveness of tumor-infiltrating lymphocyte, or TIL therapy.
FDA Approves Gammagard Liquid ERC to Treat PI, Plans to Discontinue Gammagard SD in December 2027
The U.S. Food and Drug Administration (FDA) has approved GAMMAGARD LIQUID ERC [immune globulin infusion (human)] with less than or equal to 2 µg/mL IgA in a 10% solution, the only ready-to-use liquid immunoglobulin (IG) therapy with low immunoglobulin A (IgA) content, as replacement therapy for individuals 2 years of age and older with primary immunodeficiency (PI).
Update on Measles

Once eradicated in the U.S., measles is now on the rise due to a growing mistrust of public health guidance, but it is highly preventable with vaccination.
Vaccine Advisers Recommend New RSV Vaccine for Infants
A group of outside advisers to the Centers for Disease Control and Prevention (CDC) voted 5-2 to recommend the use of Merck’s new antibody vaccine, Enflonsia (clesrovimab), that can protect babies from respiratory syncytial virus (RSV).
Flebogamma (IVIG) Effective in Treating Post-Polio Syndrome

Grifols announced positive results from its Phase II/III clinical trial evaluating the efficacy and safety of Flebogamma 5% DIF (intravenous immune globulin [IVIG]) to treat patients with post-polio syndrome, which demonstrated a significant improvement in distance walked compared to placebo.
FDA Approves Immune Checkpoint Inhibitor for Head and Neck Cancer

Pembrolizumab, an immune checkpoint inhibitor, has been approved by the U.S. Food and Drug Administration (FDA) to treat patients with resectable locally advanced head and neck squamous cell carcinoma whose tumors express PD-L1 (combined positive score [CPS] ≥1) as determined by an FDA-approved test.