Uplizna Approved to Treat IgG4-Related Disease

Amgen’s UPLIZNA has been approved by the U.S. Food and Drug Administration as the first and only treatment for adults living with Immunoglobulin G4-related disease.
GSK’s Penmenvy Meningococcal Vaccine Approved by FDA
GSK’s Penmenvy (meningococcal groups A, B, C, W and Y vaccine) has been approved by the U.S. Food and Drug Administration for use in individuals aged 10 through 25 years.
FDA Approves Dupixent for Chronic Obstructive Pulmonary Disease

The U.S. Food and Drug Administration (FDA) has expanded its approval of Dupixent to chronic obstructive pulmonary disease (COPD).
UNLOXCYT Approved to Treat Metastatic cSCC

Checkpoint Therapeutics’ UNLOXCYT (cosibelimab-ipdl) has been approved by the U.S. Food and Drug Administration to treat adults with metastatic cutaneous squamous cell carcinoma or locally advanced cSCC who are not candidates for curative surgery or curative radiation.
FDA Approves Nipocalimab to Treat Sjögren’s Disease

Johnson & Johnson’s anti-FcRn antibody nipocalimab is the first investigational therapy to be granted breakthrough therapy designation by the U.S. Food and Drug Administration as a treatment for adults with moderate-to-severe Sjögren’s disease.
Updated COVID Vaccines Approved by FDA

FDA has approved and granted emergency use authorization for updated mRNA COVID-19 vaccines (2024-2025 formula) to include a monovalent (single) component that corresponds to the Omicron variant KP.2 strain of SARSCoV-2.
FDA Approves Epinephrine Nasal Spray
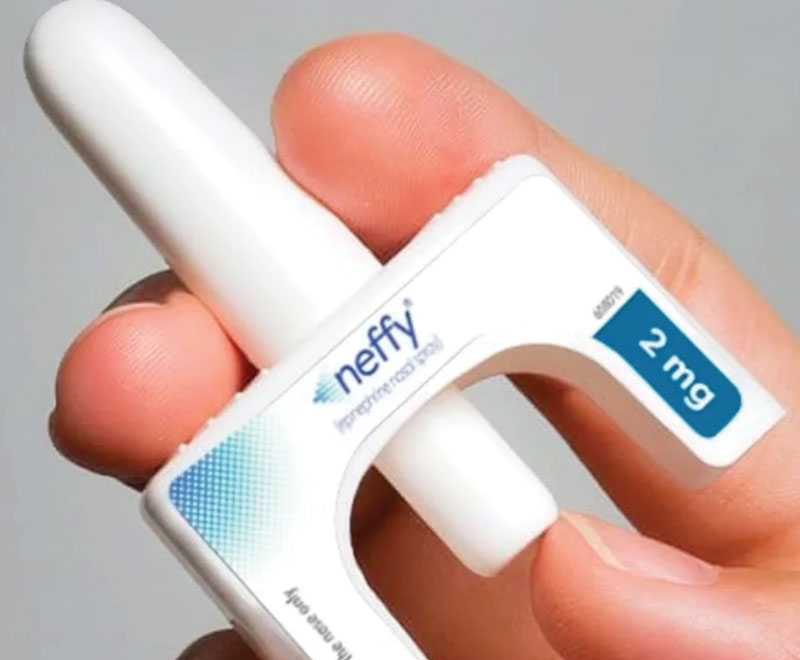
The U.S. Food and Drug Administration (FDA) has approved the first needle-free alternative to the EpiPen.
FDA Approves Additional Indication for Fibryga
FDA has approved Octapharma USA’s Fibryga (fibrinogen [human] lyophilized powder for reconstitution) for fibrinogen replacement in bleeding patients with acquired fibrinogen deficiency.
FDA Modernizes Informed Consent Guidance, Aligning with Common Rule Changes

FDA has published new draft guidance on informed consent that lines up with revisions to the Common Rule made in 2017, offering up-to-date recommendations on starting the process with the sharing of essential clinical trial information in ways that patients can understand.
FDA Approves Filspari for Rare Kidney Disease IgA Nephropathy
The U.S. Food and Drug Administration has granted full approval to Travere Therapeutics’ Filspari (sparsentan) to slow kidney function decline in adults with primary IgA nephropathy.