FDA Approves CRYOcheck Factor VIII Deficient Plasma with VWF

The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Precision BioLogic’s CRYOcheck Factor VIII (FVIII) Deficient Plasma with von Willebrand factor (VWF), opening the pathway for the company to launch the product in the U.S.
FDA Converts Leqembi to Traditional Approval to Treat Alzheimer’s

The U.S. Food and Drug Administration (FDA) has converted Leqembi (lecanemab-irmb), indicated to treat adult patients with Alzheimer’s disease, to traditional approval following a determination that a confirmatory trial verified clinical benefit.
FDA Retires Monovalent COVID-19 Vaccines

The U.S. Food and Drug Administration has amended the emergency use authorizations of the. Moderna and Pfizer-BioNTech COVID-19 bivalent mRNA vaccines to simplify the vaccination schedule for most individuals.
Pfizer’s LITFULO for Severe Alopecia Areata Approved by FDA
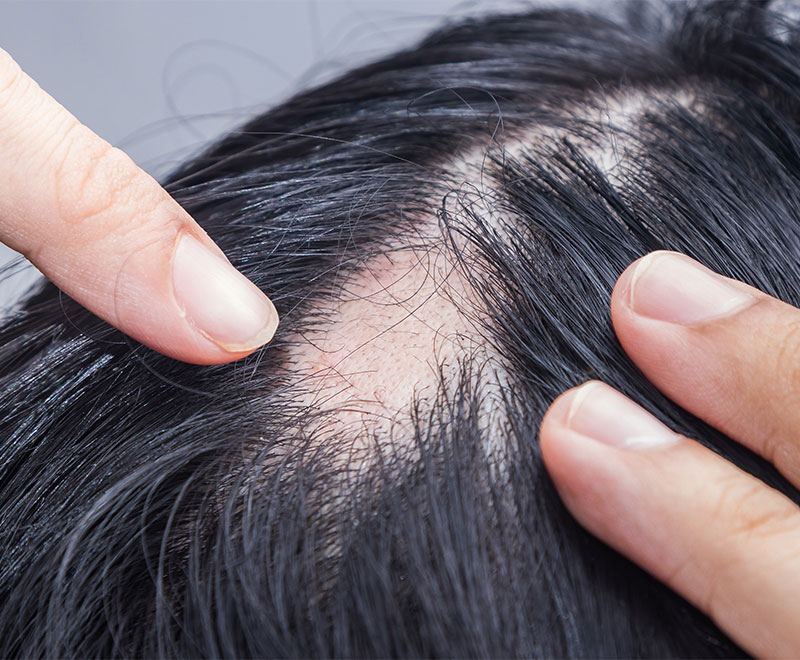
The U.S. Food and Drug Administration has approved LITFULO (ritlecitinib), a once-daily oral treatment, for individuals 12 years of age and older with severe alopecia areata.
FDA Approves Roche’s Columvi to Treat Diffuse Large B-Cell Lymphoma in Adults

The U.S. Food and Drug Administration has approved Roche’s Columvi, an antibody-based therapy chemically known as glofitamab.
Drug Granted Fast Track Approval to Treat Refractory Lupus Nephritis
The US Food and Drug Administration has granted fast track designation for Kyverna Therapeutics’ KYV-101 to treat refractory lupus nephritis.
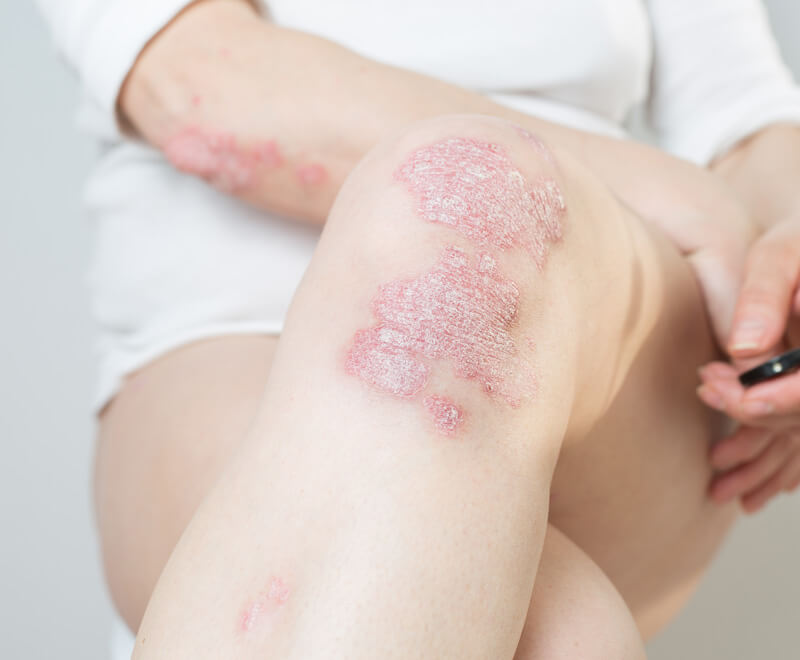
First Once-Daily Oral Plaque Psoriasis Drug Approved by FDA
The U.S. Food and Drug Administration has approved Sotyktu (deucravacitinib), a once-daily oral pill by Bristol Myers Squibb, for adults who have plaque psoriasis (a chronic, systemic, immune-mediated disease) severe enough to make them candidates for systemic therapy and phototherapy.
FDA Approves Humira Biosimilar to Treat Autoimmune Disorders
The U.S. Food and Drug Administration has approved Samsung Bioepis; Halima (adalimumab-bwwd) for the treatment of several autoimmune disorders, including rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and Crohn’s disease.
New Drugs Approved by FDA to Treat Eczema
AbbVie’s Rinvoq and Pfizer’s Cibinqo have been approved by the U.S. Food and Drug Administration (FDA) to treat moderate-to-severe atopic dermatitis.
FDA Approves Argenx’s Vyvgart to Treat MG
The U.S. Food and Drug Administration (FDA) has approved Vyvgart (efgartigi-mod) to treat generalized myasthenia gravis (gMG) in adults who test positive for the anti-acetylcholine receptor (AChR) antibody.