First Treatment Approved by FDA to Treat EGPA

GlaxoSmithKline’s Nucala (mepolizumab) has been approved by the U.S. Food and Drug Administration to treateosinophilic granulomatosis with polyangiitis (EGPA).
First Digital Pill for Mental Illness Approved by FDA

Abilify MyCite (aripiprazole tablets with sensor; Otsuka Pharmaceutical), the first digital ingestion tracking system, has been approved by the U.S. Food and Drug Administration.
FDA Issues Product Advisory for CSL Behring’s AlbuRx

CSL Behring noted the potential for fading print with more effect on the expiration dating on the patient tear-off portion of the vial label.
New Drug Approved by FDA to Treat Acute Myelogenous Leukemia
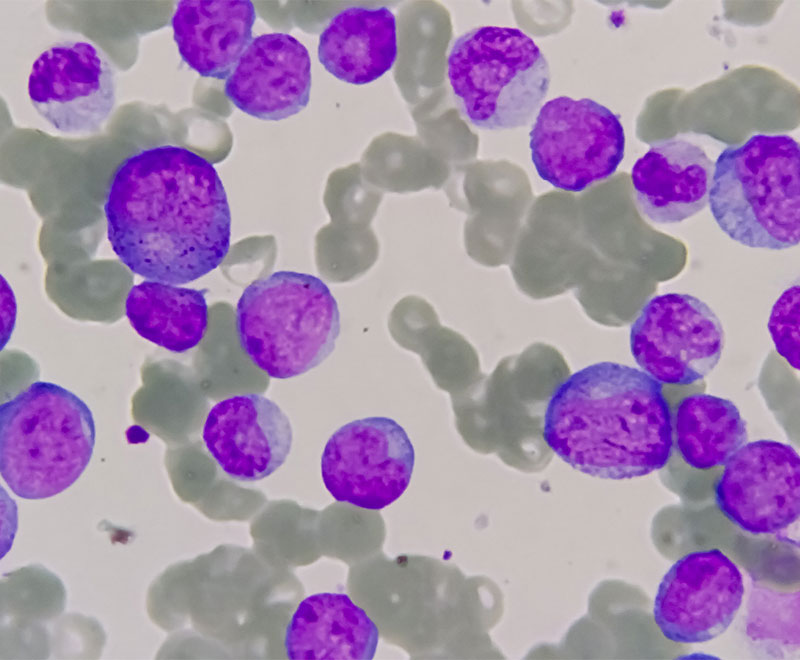
The U.S. Food and Drug Administration has approved Agios Pharmaceuticals’ Idhifa (enasidenib) to treat acute myelogenous leukemia.
FDA Approves First Subcutaneous Therapy to Treat HAE
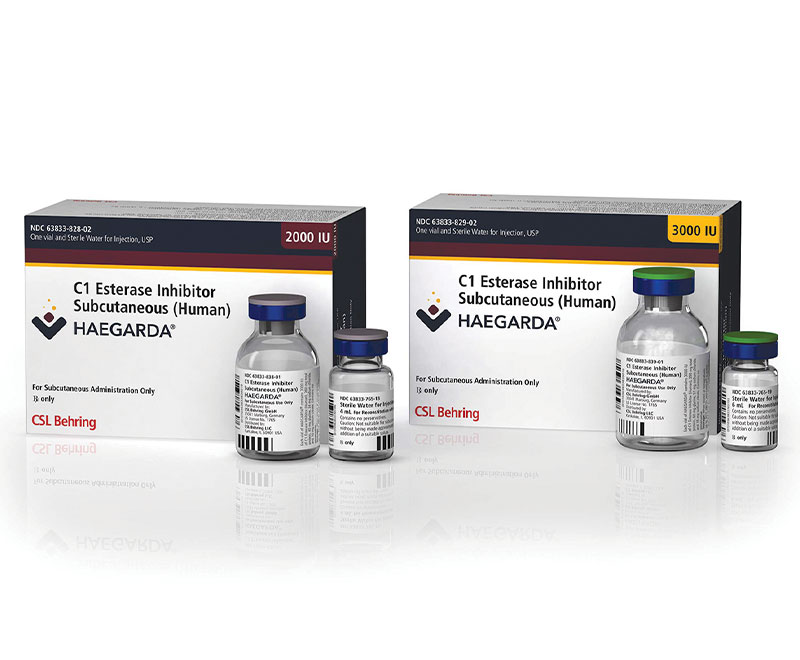
The U.S. Food and Drug Administration has approved CSL Behring’s Haegarda (C1 esterase inhibitor subcutaneous [human]), the first and only subcutaneous therapy indicated for routine prophylaxis to prevent hereditary angioedema attacks in adolescent and adult patients.
CSL Behring ’s Privigen Now Approved to Treat CIDP
The U.S. Food and Drug Administration (FDA) has approved Privigen (immune globulin intravenous [human] 10% liquid) to treat adults with chronic inflammatory demyelinating polyneuropathy (CIDP) to improve neuromuscular disability.
FDA Approves Mavyret to Treat Adults with Chronic Hepatitis C
The U.S. Food and Drug Administration has approved AbbieVie Inc.’s Mavyret (glecaprevir and pibrentasvir) to treat adults with certain types of hepatitis C.
Kedrion Biopharma and Kamada Receive FDA Approval for KEDRAB
The U.S. Food and Drug Administration has approved Kedrion Biopharma’s and Kamada’s KEDRAB (rabies immune globulin [human]) for passive, transient postexposure prophylaxis of rabies infection.
Cutting-Edge Pediatric Cancer Therapy Approved by FDA

Novartis’ Kymriah (tisagenlecleucel) has been approved by the U.S. Food and Drug Administration to treat pediatric acute lymphoblastic leukemia.
FDA Approves First Drug to Treat Giant Cell Arteritis
The U.S. Food and Drug Administration has approved Roche’s Actemra (tocilizumab), the first treatment for adult patients with giant cell arteritis.