CMS Launches Voluntary Bundled Payment Model

The Centers for Medicare and Medicaid Services Center for Medicare and Medicaid Innovation has launched a new voluntary bundled payment model called Bundled Payments for Care Improvement Advanced.
Hike Proposed for Medicare Payments in 2019

The Centers for Medicare and Medicaid Services has proposed a 1.84 percent increase in 2019 Medicare payments to health insurers that manage Medicare Advantage insurance plans for more than 20 million elderly and disabled people.
HHS Proposed Significant Cuts to Medicare Payments for 340B Drugs

The U.S. Department of Health and Human Services has proposed 2018 updates to the Medicare hospital outpatient prospective payment system to decrease Medicare Part B payments to hospitals for 340B drugs by almost 30 percent.
Senate Passes CHRONIC Care Act of 2017

The U.S. Senate passed the Creating High-Quality Results and Outcomes Necessary to Improve Chronic(CHRONIC) Care Act of 2017, which aims to improve care for seniors with chronic conditions.
Congress Passes Bills Extending CHIP Funding

The House Energy and Commerce Committee and the Senate Finance Committee passed bills to extend for five years the federal funding forChildren’s Health Insurance Program.
New Rules Narrow ACA’ s Contraception Mandate

New rules issued by the U.S. Department of Health and Human Services give employers more leeway to withhold birth control coverage on religious grounds.
FDA Approves and CDC Recommends Shingrix Vaccine to Prevent Shingles
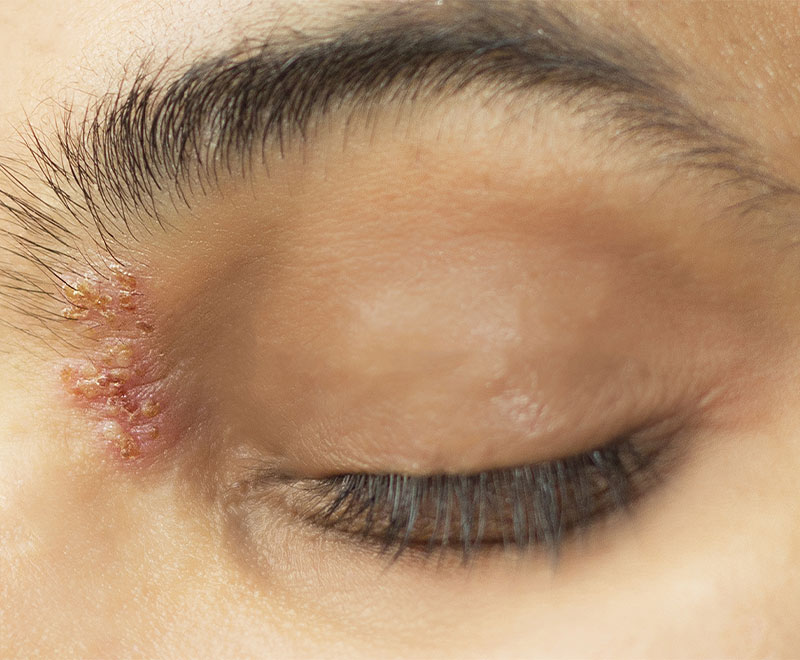
GlaxoSmithKline (GSK) has receivedU.S. Food and Drug Administration approval for its Shingrix vaccine to prevent shingles (herpes zoster) in patients 50 years and older.
Supreme Court Rules in Favor of Faith-Based Hospitals’ Exemption from ERISA

The U.S. Supreme Court ruled that faith-based hospitals’ pension plans qualify for the “church plan” exemption from the Employee Retirement Income Security Act (ERISA).
More Small Practices Exempt from MACRA Under New Draft Rule

The Centers for Medicare and Medicaid would exempt physician practices with less than $90,000 in Medicare revenue or fewer than 200 unique Medicare patients per year from complying with the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
Drug Companies Can Be Fined for Overcharging Hospitals Under 340B Drug Discount Program
Under a new rule, the Health Resources and Services Administration of the Department of Health and Human Services can issue fines of up to $5,000 for each incident to drug manufacturers that knowingly and intentionally overcharge 340B hospitals for drugs purchased under the program.