Thirty Years On, IVIG Clinical Research Keeps Rolling
Thirty years after its quiet introduction, unabated global research interest in IVIG reflects just how much remains unknown about its therapeutic potential.
Dermatomyositis with Isolated Severe Skin Lesions Responds to IVIG Therapy
Investigators concluded that IVIG may be an effective and safe treatment for DM with isolated skin involvement.
The Physician’s Role in Immune Globulin Reimbursement

Attention to meeting payer requirements is good for the bottom line.
Immune Globulin: Each Product Is Unique
While all IG products are comparably effective, they also have relevant differences that determine their tolerability by patients.
CDC Updates VariZIG Use Recommendations
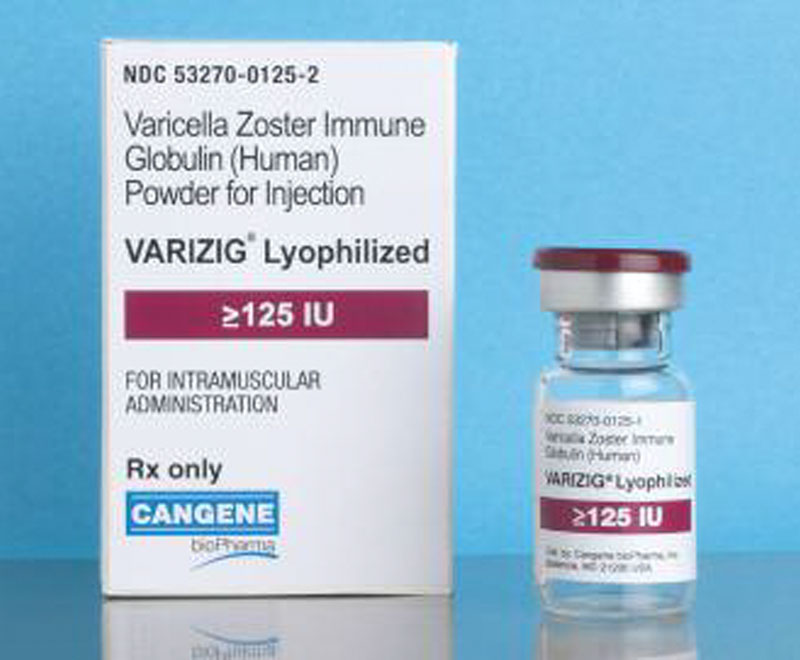
The Centers for Disease Control and Prevention has updated its recommendations for the use of VariZIG.
New Vial Size Approved for CSL Behring’s Hizentra

The U.S. Food and Drug Administration has approved a 10 g (50mL) vial size for Hizentra, immune globulin subcutaneous (human).
New Boxed Warning Added to Immune Globulin Products

The U.S. Food and Drug Administration has instructed manufacturers to add information to the current boxed warning in the labeling of all intravenous immune globulin (IVIG) products, and add a similar standardized boxed warning for all subcutaneous and intramuscular IG products.
Intravenous Immunoglobulin Sharply Reduces Relapse Rate in a Series of Patients with Neuromyelitis Optica
With the objective of evaluating the safety and tolerability of intravenous immune globulin (IVIG) as a treatment for neuromyelitis optica (NMO), a team of Spanish investigators administered IVIG (0.7 g/kg body weight for three days) every two months to eight patients meeting Wingerchuk’s revised diagnostic criteria for the disorder.
Short Course of IVIG Slows Early Alzheimer’s

Results from a new study in patients with early Alzheimer’s disease showed a short course of intravenous immune globulin slows the disease’s progression.
IVIG as Effective as Plasma Exchange in Treating Guillain Barré Syndrome

A meta-analysis showed IVIG was as effective as plasma exchange in reducing Guillain Barré syndrome disability scores, with no significant difference in adverse events.