IVIG Improves Live Birth Rate in Women with Immune Conditions and Recurrent Pregnancy Loss: Review and Meta-Analysis

Dutch investigators conducted a systematic review and meta-analysis of studies on the effectiveness of IVIG treatment of this specific population.
IVIG May Reverse Symptoms of Down Syndrome Regression Disorder

A new study shows treatment with IVIG shows promise for children and young adults with Down syndrome regression disorder (DSRD).
High-Dose IVIG Effective in Painful Diabetic Polyneuropathy Resistant to Conventional Treatments
Italian investigators conducted a trial to assess the safety and efficacy of IVIG in treatment-resistant painful diabetic polyneuropathy (DPN).
IVIG Plus Glucocorticoids Superior to IVIG Alone for MIS-C Associated with COVID-19: Review and Meta-Analysis

A new systematic review and meta-analysis conducted by U.S. and Nepalese collaborators supports the use of IVIG with glucocorticoids compared to IVIG alone.
Many Clinically Stable CIDP Patients Can Safely Stop IVIG Maintenance Treatment

While intravenous immune globulin (IVIG) therapy is efficacious for patients with chronic inflammatory demyelinating polyneuropathy (CIDP), the lack of biomarkers for disease activity makes the need for ongoing treatment difficult to assess.
IG Treatment May Reduce Acute Exacerbations of COPD
Observational studies suggest immune globulin (IG) treatment may reduce the frequency of acute exacerbations of chronic obstructive pulmonary disease (AECOPD).
Fact or Fiction: Debunking the Myths Surrounding IG Therapy Improves Patient Outcomes
Experts set the record straight about common misunderstandings regarding IVIG and SCIG products, their administration and possible reactions.
IVIG Plus Glucocorticoids Effective for Treating COVID-19 Pediatric Syndrome

A large multicenter clinical trial has found intravenous immune globulin (IVIG) plus glucocorticoids may be better than IVIG alone for treating multisystem inflammatory syndrome in children (MIS-C) caused by COVID-19.
The Current Challenge of Immune Globulin Access
Understanding the factors contributing to the current shortage of immune globulin could help to address a crisis that threatens dire consequences for patients.
Against the Storm: High-Dose IVIG as an Immunoregulatory Treatment Strategy for Severe COVID-19
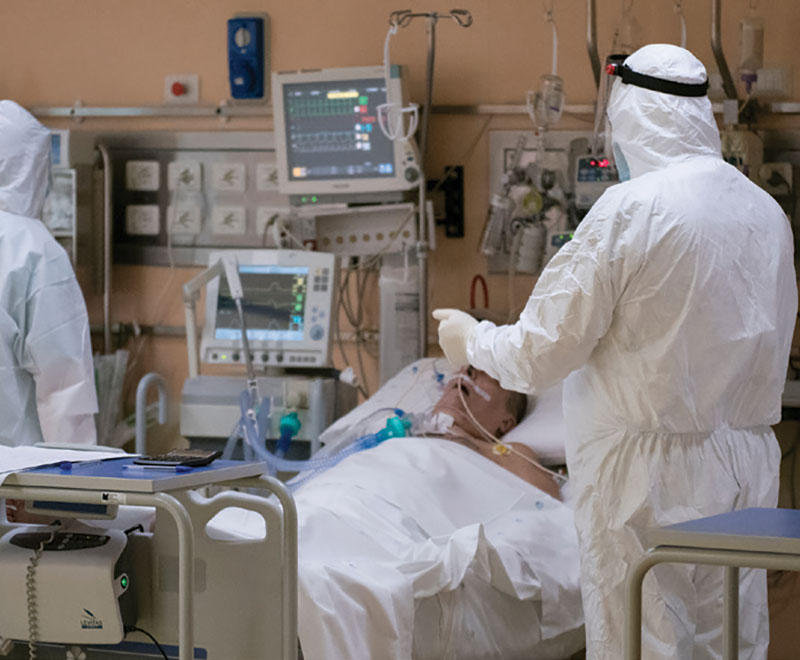
While there may be no “magic bullet” to treat cytokine storm, one widely used immunomodulatory agent in particular — polyclonal intravenous immune globulin (IVIG) purified from healthy donor plasma — is distinguished by the simple fact that it is anything but a narrowly targeted treatment.