FDA Grants wilate Orphan Drug Exclusivity

The U.S. Food and Drug Administration (FDA) has granted orphan drug exclusivity for Octapharma’s wilate, von Willebrand factor/coagulation factor VIII complex (human) lyophilized powder for solution for intravenous injection, for routine prophylaxis to reduce the frequency of bleeding episodes in adults and children 6 years of age and older with von Willebrand disease (VWD).
How FDA Is Working to Accelerate Rare Disease Treatments

Through regulatory pathways and patient engagement, FDA is helping to advance treatment innovations for rare diseases.
Cryoprecipitate, Fibrinogen Concentrates and New Pathogen Reduced Cryo Product Vie for Use in Massive Hemorrhage

Does the shorter preparation time for fibrinogen concentrates make them a reasonable option in lieu of IFC in defined patient populations experiencing massive hemorrhage?
Caring for Mental Health in a World of Uncertainty

Post-pandemic America is facing a mental health crisis, but collaboration and creativity in healthcare aim to improve the nation’s mental wellness.
Solving Clinical Trial Delays by Accelerating Patient Recruitment

Enrolling patients in clinical trials is a slow and costly process, but software companies are working to address the problem.
Mitigating AI Risks for Consumer Health Misinformation

While agencies race to put in place regulations for using artificial intelligence to reduce the spread of health misinformation, healthcare providers can help to correct this by engaging with their patients.
Myths & Facts: Sleep Disorders

Sleep disorders are responsible for a host of physical and mental health conditions, making it essential to dispel the myths surrounding them and to promote education and awareness about the importance of sleep health.
Update on Malaria

While malaria is no longer endemic in the United States, the mosquito-borne disease can still affect those who live in or travel to tropical areas of the globe.
First Do No Harm: Increasing Patient Safety

Patient safety is at the heart of healthcare, so it’s imperative to put in place systems and regulations to reduce patient harm.
Update on Molluscum Contagiosum
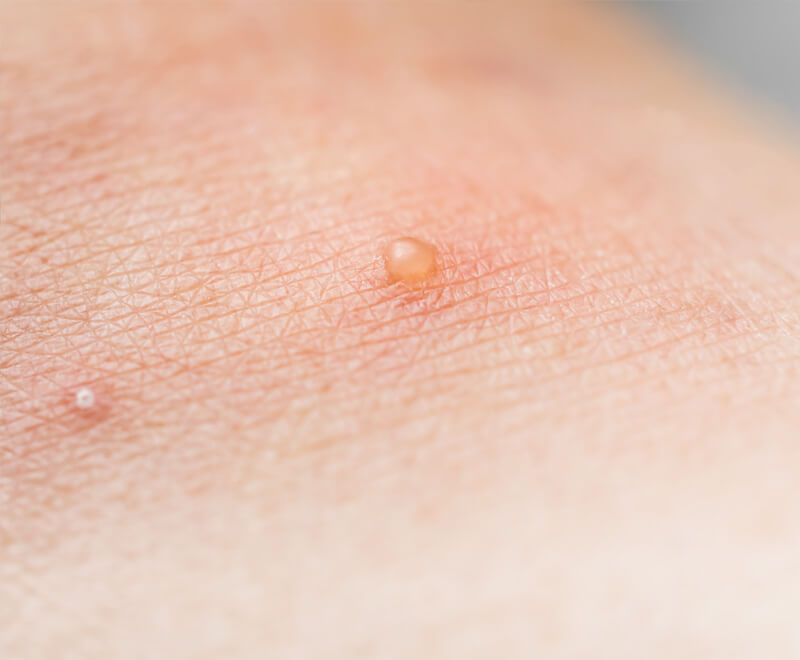
Though mostly benign, this bothersome skin disease could be dangerous for those with a compromised immune system. But while an effective, FDA-approved treatment specially formulated for the highly contagious poxvirus has eluded the disease — that’s no longer the case.