Second Octapharma Octagam 10% Manufacturing Site Approved by FDA
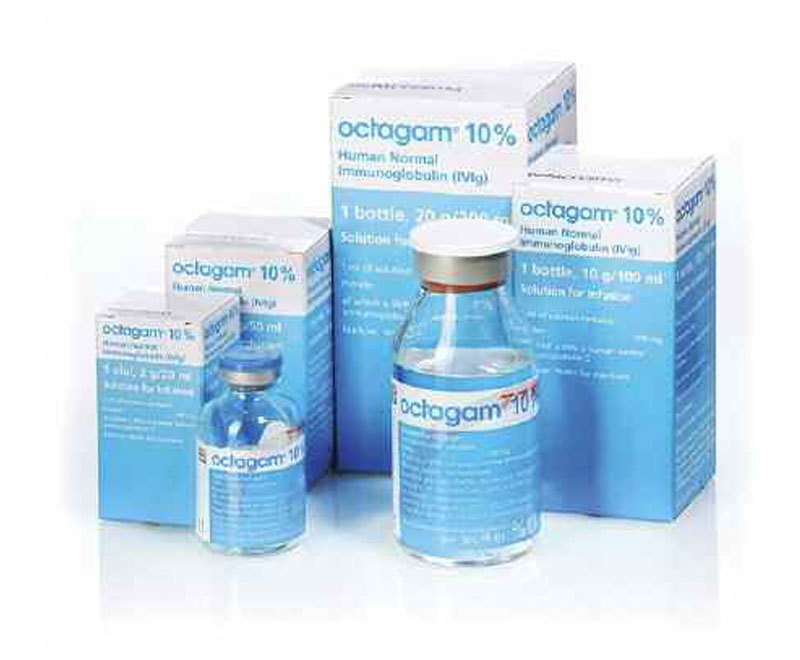
The U.S. Food and Drug Administration(FDA) has approved Octapharma’s manufacturing facility in Vienna, Austria, for the production of Octagam 10% (immune globulin intravenous [human] 10% [100mg/mL] liquid preparation).
Should There Be a ‘Right to Try’?
The debate surrounding right-to-try laws to allow patients access to potentially life-saving drugs hinges on safety and ethical concerns.
Biosimilars: The Race for Approval

As the approval of biosimilars looms, debate continues over whether they should be substituted for biopharmaceuticals, how to legislate them and how they should be named.
Exploring the Power of IVIG
Clinical studies using intravenous immune globulin therapy are breaking new ground when it comes to treating chronic disease; promising results are being seen in patients suffering from Alzheimer’s, autism and even diabetes.
Supreme Court Upholds Exemption to ACA’s Contraception Coverage Requirement

In June, the U.S. Supreme Court voted 5-4 to allow a key exemption to the Affordable Care Act’s contraception coverage requirements, meaning closely held, for-profit businesses can assert a religious objection.
Immune Globulin: Controlling Supply and Demand

While the healthcare industry is currently experiencing an oversupply of the lifesaving immune globulin therapy, with demand growing at 6 percent to 8 percent a year, is it possible another shortage looms large?
Subcutaneous Immune Globulin: New Therapeutic Uses Beyond Primary Immunodeficiency?

While the intravenous route has been the standard method of IG therapy for autoimmune and other neuromuscular disorders, recent studies show that the subcutaneous route is both as effective and more preferred by patients.
New Therapy Requires Less Frequent Dosing for Hemophilia B Patients

Interim Phase II/III and III findings of a study conducted by CSLBehring demonstrate an improved pharmacokinetic profile of recombinant fusion protein linking coagulation factor IX with recombinant albumin (rIX-FP) among hemophiliaB patients in all age groups.
rFVIII Treatment for Hemophilia A Receives Positive Results
Baxter’s Phase III clinical trial of BAX855, an investigational, extended half-life recombinant factor VIII (rFVIII) treatment for hemophilia A based on ADVATE (Antihemophilic Factor[Recombinant]), has met its primary endpoint in reducing annualized bleeding rates (ABR) in the prophylaxis arm compared with the on-demand arm.
FDA Approves Tivorbex

FDA has approved Iroko Pharmaceuticals’ Tivorbex (indomethacin), a low-dose painkiller for adult patients.