Emicizumab Prophylaxis Efficacious and Well-Tolerated in Infants with Severe Hemophilia A Without Inhibitors

The Phase IIIb study of emicizumab shows promise for reducing risk of spontaneous and traumatic bleeds in infants with hemophilia A.
FDA Approves New Safety Labeling for Opioid Medications

FDA approved and implemented updated warning labels for all opioid prescriptions.
FDA Approves First Cell Therapy to Treat Melanoma
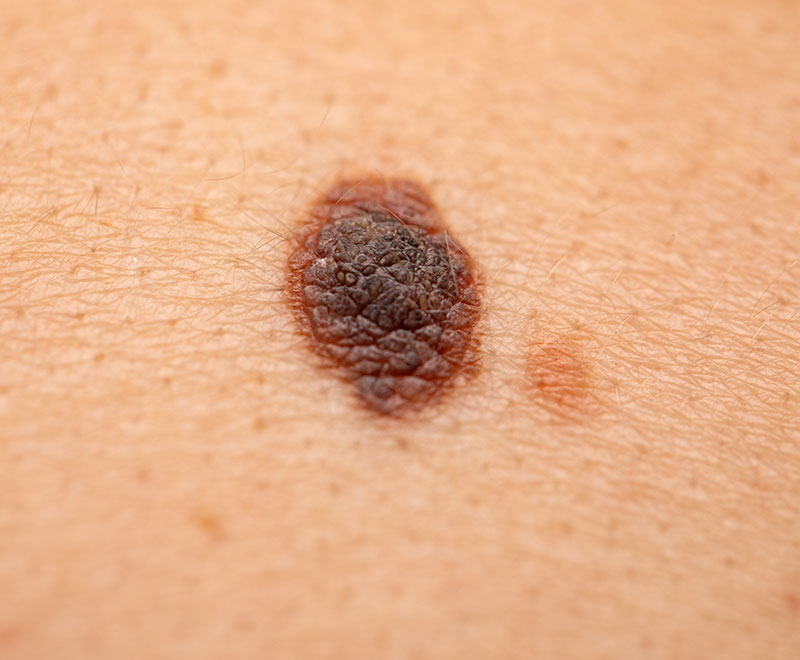
FDA approved Amtagvi, the first cellular therapy indicated for the treatment of adult patients with unresectable melanoma or metastatic melanoma that previously has been treated with other therapies.
Third Humira Biosimilar Is Approved by FDA

FDA has approved Alvotech’s Simlandi (adalimumab-ryvk) as the third interchangeable Humira biosimilar.
New Guidance Recommends All Federal Facilities Have Access to Naloxone
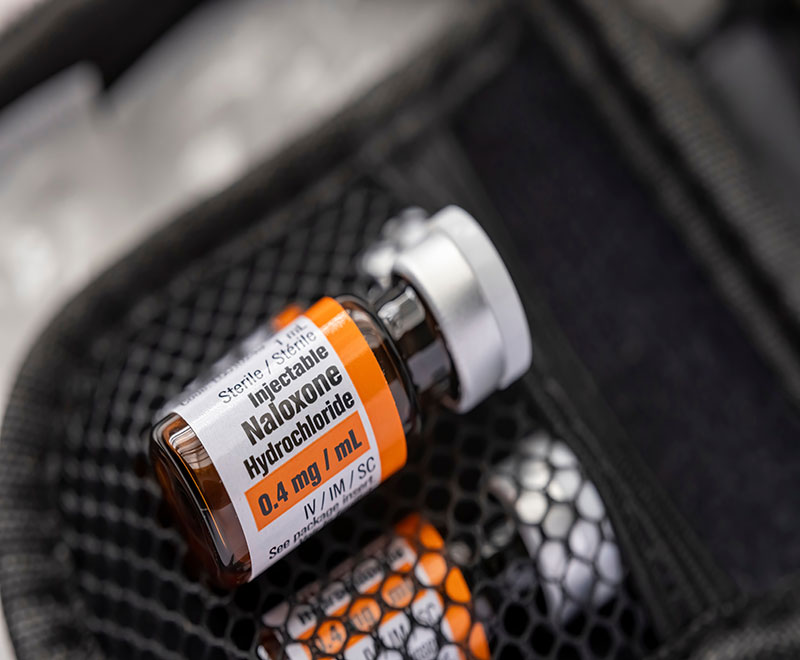
The U.S. Department of Health and Human Services and the General Services Administration have announced new guidance recommending that all federal facilities across the nation include overdose reversal medications in their safety stations on site.
FDA Approves Zepbound to Treat Obstructive Sleep Apnea

The U.S. Food and Drug Administration has authorized the use of Eli Lilly & Co.’s Zepbound for adults with obesity and moderate to severe obstructive sleep apnea, a common condition where a person struggles to breathe properly during sleep.
First Treatment Approved for Genetic Clotting Disorder
The U.S. Food and Drug Administration has approved a recombinant ADAMTS13 protein product (Adzynma) as a prophylactic or on-demand enzyme replacement therapy for patients with congenital thrombotic thrombocytopenic purpura.
First Vaccine for Mosquito-Born Viral Disease Is Approved by FDA
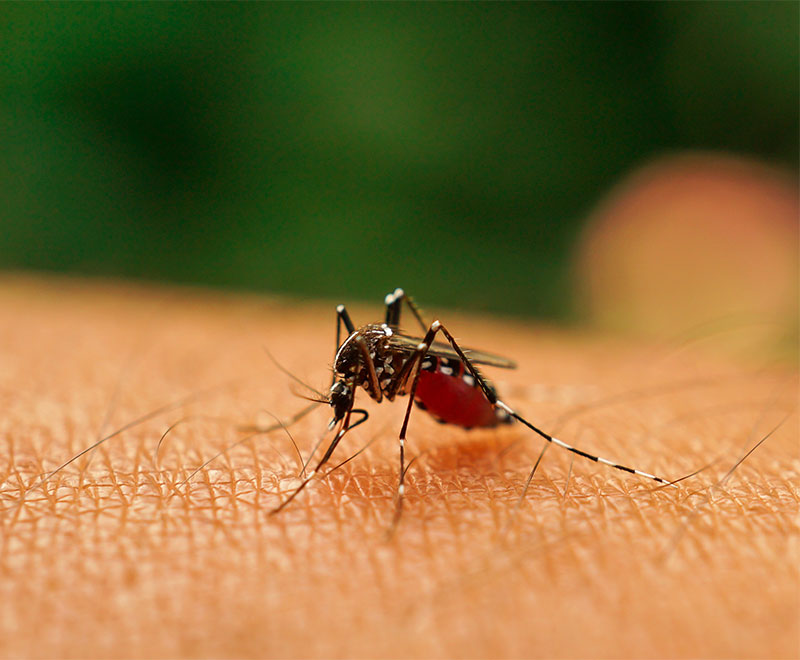
The U.S. Food and Drug Administration has approved IXCHIQ, Valneva’s single-dose, live-attenuated vaccine indicated for the prevention of disease caused by chikungunya virus in individuals 18 years of age and older who are at increased risk of exposure to CHIKV.
FDA Approves Zepbound as a New Weight-Loss Drug

The U.S. Food and Drug Administration has approved Eli Lilly’s tirzepatide medication, which is branded as Mounjaro for diabetes, under a new brand for weight loss as well.
Efgartigimod Efficacious in Treating Chronic Immune Thombocytopenia
A new study shows efgartigimod significantly increased sustained platelet count responses compared with placebo in patients with chronic immune thrombocytopenia, including those who had received multiple previous immune thrombocytopenia therapies.