Complement C5 Inhibitor Zilucoplan Is Approved by FDA to Treat MG

UCB Pharma’s investigational agent zilucoplan, a complement C5 inhibitor, to treat patients with myasthenia gravis has been approved by the U.S. Food and Drug Administration under the market name Zilbrysq.
IVIG Reduces Infection Risk During Anti-BCMA Treatment for MM

Data suggests the bispecific BCMA-targeted antibody teclistamab may lead to grade 3 to 5 infections in as many as 44.8 percent of multiple myeloma patients, and hypogammaglobulinemia was noted in nearly 75 percent of patients.
FDA Approves Antibody to Protect Infants Against RSV
The U.S. Food and Drug Administration (FDA) has approved nirsevimab to protect newborns from respiratory syncytial virus (RSV).
FDA Approves First Postpartum Depression Oral Treatment

The U.S. Food and Drug Administration has approved Zurzuvae (zuranolone), the first oral medication indicated to treat postpartum depression (PPD) in adults.
First Over-the-Counter Contraceptive Pill Gets FDA Approval
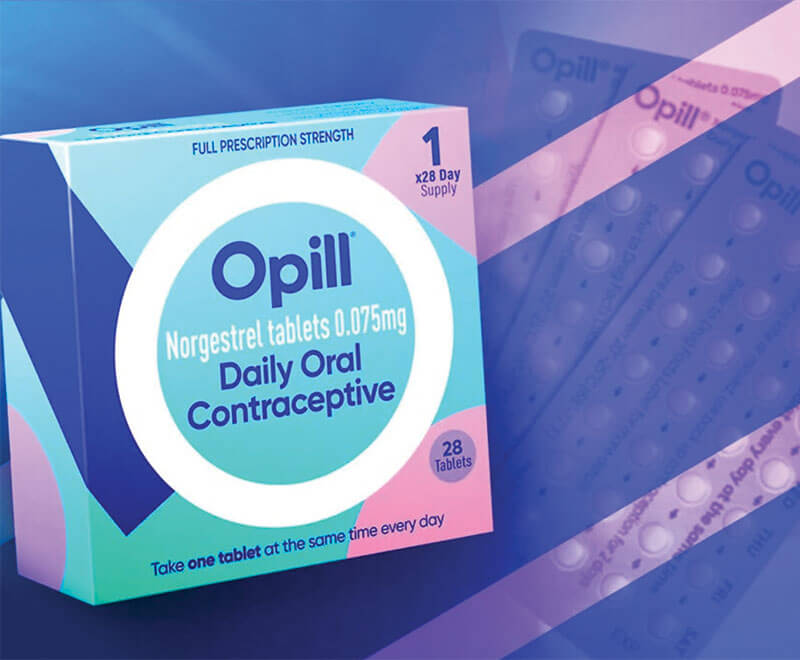
The U.S. Food and Drug Administration has approved the birth control pill Opill (norgestrel), manufactured by Perrigo, to be available over-the-counter — the first nonprescription birth control pill in the United States.
FDA Approves Pfizer’s ABRYSVO Vaccine for the Prevention of RSV

Pfizer’s ABRYSVO, a respiratory syncytial virus (RSV) vaccine, has been approved by the U.S. Food and Drug Administration for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth up to 6 months of age by active immunization of pregnant women at 32 through 36 weeks gestational age.
FDA Approves Biosimilar to ACTMRA

The U.S. Food and Drug Administration has approved Biogen’s tocilizumab-bavi (TOFIDENCE), a biosimilar option referencing ACTMRA, to treat moderately to severely active rheumatoid arthritis (RA), polyarticular juvenile idiopathic arthritis (PJIA) and systemic juvenile idiopathic arthritis (JIA).
FDA Converts Leqembi to Traditional Approval to Treat Alzheimer’s

The U.S. Food and Drug Administration (FDA) has converted Leqembi (lecanemab-irmb), indicated to treat adult patients with Alzheimer’s disease, to traditional approval following a determination that a confirmatory trial verified clinical benefit.
Pfizer’s LITFULO for Severe Alopecia Areata Approved by FDA
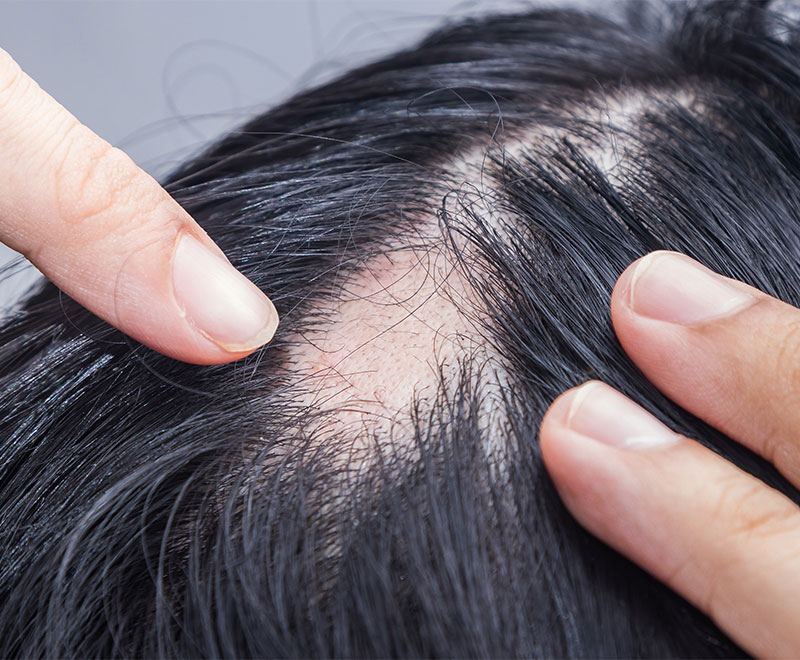
The U.S. Food and Drug Administration has approved LITFULO (ritlecitinib), a once-daily oral treatment, for individuals 12 years of age and older with severe alopecia areata.
First Subcutaneous Injectable Approved to Treat Generalized Myasthenia Gravis
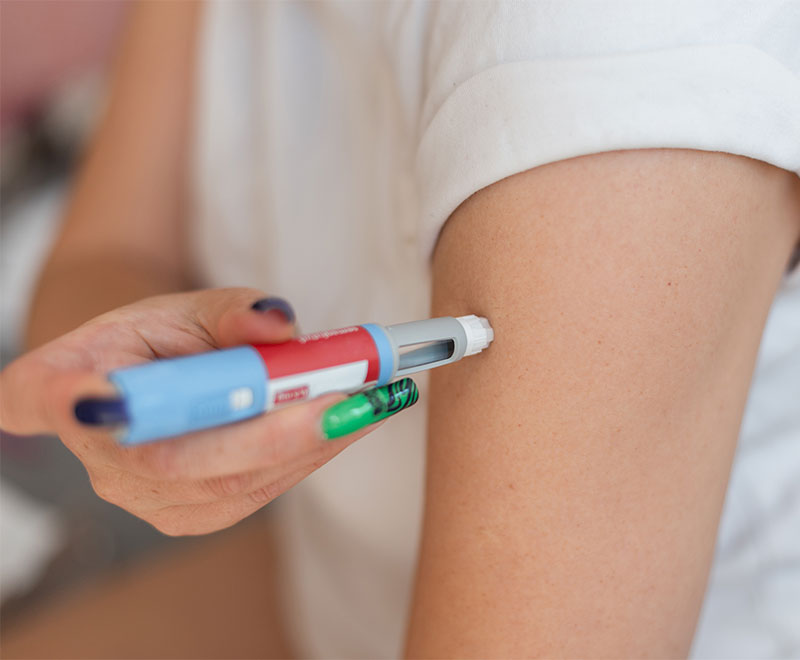
The subcutaneous formulation of argenx’s Vyvgart has been approved by the U.S. Food and Drug Administration to treat generalized myasthenia gravis.