FDA Approves Celltrion’s Steqeyma to Treat Autoimmune Diseases

Celltrion has received U.S. Food and Drug Administration approval for its autoimmune disease treatment drug Steqeyma, a biosimilar of Johnson & Johnson’s Stelara (ustekinumab).
Immuneering Granted FDA Fast Track Designation for IMM-1-104 in Advanced Melanoma
The U.S. Food and Drug Administration (FDA) has granted fast track designation for its lead clinical-stage program, IMM-1-104, as a treatment for patients with unresectable or metastatic NRAS-mutant melanoma who have progressed on or are intolerant to PD-1/PD-L1-based immune checkpoint inhibitors.
New Blood Test for Cancer Patients Makes Treatment Safer and More Effective
Scientists from RMIT University and the Doherty Institute in Australia have developed a new blood test that could screen cancer patients to help make their treatment safer and more effective.
UNLOXCYT Approved to Treat Metastatic cSCC

Checkpoint Therapeutics’ UNLOXCYT (cosibelimab-ipdl) has been approved by the U.S. Food and Drug Administration to treat adults with metastatic cutaneous squamous cell carcinoma or locally advanced cSCC who are not candidates for curative surgery or curative radiation.
FDA Observes Serious Liver Injury After Treatment with Ocaliva
FDA identified cases of serious liver injury among patients being treated for primary biliary cholangitis with Ocaliva who did not have cirrhosis of the liver.
Trial Shows Experimental Cancer Drug Helped Reduce Risk of Death in Common Blood Cancer
Results of a trial of GSK’s experimental cancer drug Blenrep showed that the drug used in combination with other treatments reduced the risk of death by 42 percent in multiple myeloma, at or after first relapse, compared to an existing treatment.
COVID-19 Vaccine May Reduce Risk of Developing Long COVID

A new study published in the journal Vaccine, shows that receiving a COVID-19 vaccine could reduce the risk of developing long COVID if it’s administered five months before an infection.
FDA Approves YESINTEK to Treat Autoimmune Diseases

Biocon Biologics has received U.S. Food and Drug Administration (FDA) approval for YESINTEK, a monoclonal antibody for the treatment of autoimmune conditions, including Crohn’s disease, ulcerative colitis, plaque psoriasis and psoriatic arthritis. Y
Study Reveals Best Time to Get RSV Vaccine During Pregnancy
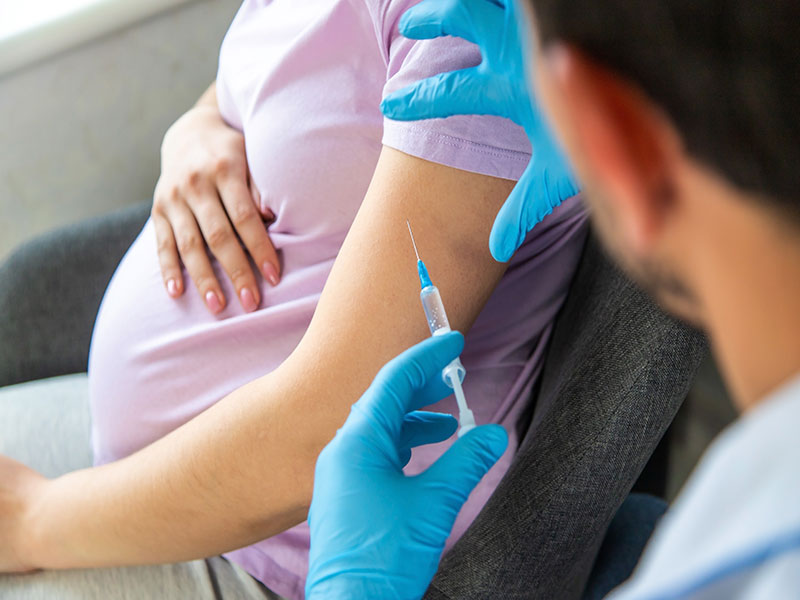
New research led by investigators at Mass General Brigham suggests vaccination closer to 32 weeks of pregnancy, rather than the current guidelines of between 32 and 36 weeks, will better protect pregnant women’s newborns against respiratory syncitial virus (RSV).
FDA Approves Nipocalimab to Treat Sjögren’s Disease

Johnson & Johnson’s anti-FcRn antibody nipocalimab is the first investigational therapy to be granted breakthrough therapy designation by the U.S. Food and Drug Administration as a treatment for adults with moderate-to-severe Sjögren’s disease.