FDA Approves Biosimilar to Rheumatoid Arthritis Drug
Amgen’s biosimilar to Johnson & Johnson’s rheumatoid arthritis drug, Remicade, has been approved by the U.S. Food and Drug Administration.
Study of Kevzara from Severe to Critical COVID-19 Patients

Following a review by the Independent Data Monitoring Committee (IDMC) of preliminary results from the Phase II portion of an ongoing Phase II/III trial evaluating Kevzara (sarilumab), the trial was amended so only critical patients continue to be enrolled to receive Kevzara 400 mg or placebo.
Clinical Trial Will Assess IVIG for Treating COVID-19

Octapharma USA is supporting a new investigator-initiated clinical trial led by George Sakoulas, MD, of Sharp Memorial Hospital in San Diego, Calif., focused on treating the most critical coronavirus patients who are experiencing respiratory failure who become ventilator dependent.
Novartis Initiates Phase III Trial of Ilaris to Treat COVID-19 Patients with Pneumonia
Novartis has initiated a Phase III clinical trial to examine the efficacy of utilizing canakinumab (Ilaris), an interleukin (IL)-1β blocker, to treat a type of severe immune overreaction called cytokine release syndrome (CRS) in people with COVID-19 pneumonia.
Central Repository Created for SARS-CoV 2 Clinical Trials

Inato, a marketplace that helps biopharmaceutical companies increase the pool of available patients engaged in clinical trials, has unveiled its anticovid platform, a comprehensive, central repository for all existing clinical trials for SARS-CoV 2 (the virus that causes COVID-19).
Study Suggests MMR Vaccine May Be Linked with Fewer Deaths from COVID-19
Epidemiological data suggests populations with the highest measles-mumps-rubella (MMR) vaccination rates often have the fewest deaths from COVID-19.
Waiver Allows Medicare Patients to Receive Free Telehealth Services

The Centers for Medicare and Medicaid Services (CMS) has broadened access to Medicare telehealth services so beneficiaries can receive a wider range of services from their doctors without having to travel to a healthcare facility.
FDA Campaign Designed to Help Consumers Use New Food Label
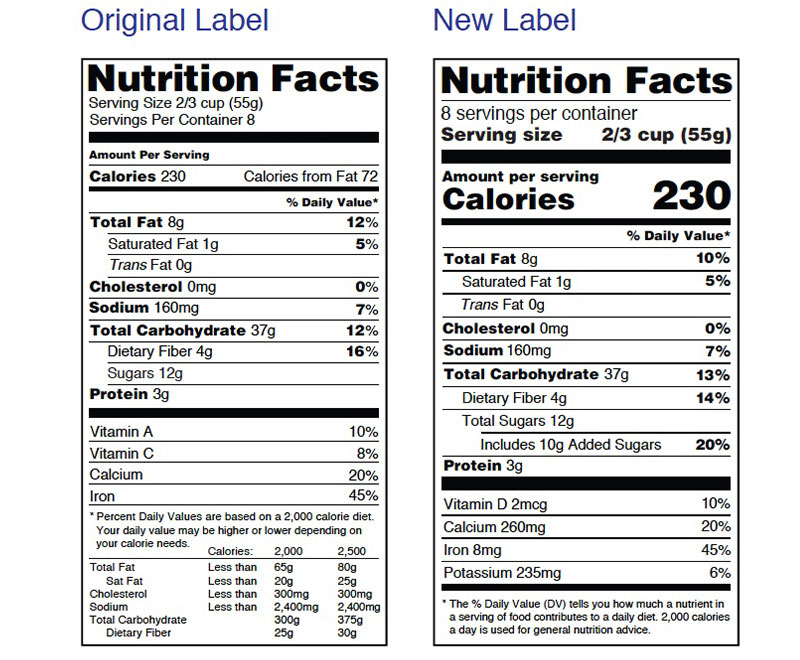
The U.S. Food and Drug Administration (FDA) has launched a campaign to help consumers use the new Nutrition Facts label that appears on packaged foods to maintain healthy dietary practices.
Finalized Rule Gives Patients More Control Over Their Health Data

In March, the U.S. Department of Health and Human Services (HHS) finalized two rules that give patients unprecedented safe and secure access to their health data.
Coronavirus Relief Funds to Pay for Care for Uninsured
The U.S. Department of Health and Human Services (HHS) is using a $100 billion hospital and provider relief fund to pay hospitals at Medicare rates for uncompensated COVID-19 care for uninsured individuals.